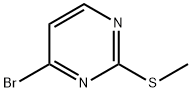
2-METHYLTHIO-4-BROMOPYRIMIDINE synthesis
- Product Name:2-METHYLTHIO-4-BROMOPYRIMIDINE
- CAS Number:959236-97-6
- Molecular formula:C5H5BrN2S
- Molecular Weight:205.08
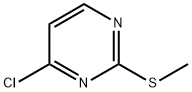
49844-90-8

959236-97-6
General procedure for the synthesis of 2-methylthio-4-bromopyrimidine from 2-methylthio-4-chloropyrimidine: Bromotrimethylsilane (23 mL, 174.31 mmol) was added slowly and dropwise to an anhydrous acetonitrile (240 mL) solution of 4-chloro-2-methylthio-pyrimidine (2.9 g, 25 mmol) under argon protection. The reaction mixture was stirred in an oil bath at 40 °C for 30 h, during which the progress of the reaction was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched by slow addition of saturated sodium bicarbonate solution (250 mL). The aqueous phase was extracted with ethyl acetate (3 × 100 mL), and the organic phases were combined and dried over anhydrous sodium sulfate. After filtration to remove the desiccant, the organic phase was concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography with the eluent dichloromethane/ethyl acetate (9:1, v/v). The target fraction was collected and the solvent was evaporated under reduced pressure to afford the target compound 2-methylthio-4-bromopyrimidine as a colorless oil in 96% yield (4.66 g). The structure of the product was confirmed by 1H NMR, 13C NMR and HPLC: 1H NMR (CDCl3) δ 2.56 (s, 3H, SCH3), 7.16 (d, 1H, J = 5.2 Hz, H-5), 8.26 (d, 1H, J = 5.2 Hz, H-6); 13C NMR (CDCl3) δ 13.5 (SCH3), 116.2 (C-5), 140.3 (C-6), 158.7 (C-4), 162.4 (C-2); HPLC Rt = 3.11 min.
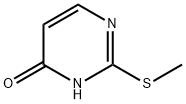
5751-20-2
305 suppliers
$14.00/1g

959236-97-6
105 suppliers
$14.00/250mg
Yield:959236-97-6 83%
Reaction Conditions:
with phosphorus(V) oxybromide in acetonitrile at 80; for 5 h;
Steps:
695.1 4-bromo-2-(methylthio)pyrimidine
To a stirred solution of 2-(methylthio)pyrimidin-4(3H)-one (5 g, 35.21 mmol, leq) in ACN (lOOmL) was added POBr3 (12.1 g, 42.3 mmol, 1.2 eq) at RT, then the reaction mixture was heated to 80°C for 5h. Monitored by TLC, the reaction mixture was cooled to RT and quenched in ice cold water then extracted with EtOAc (2X100mL). The combined organic layer was dried over Na2SC>4 and concentrated to crude compound. The crude compound was purified by column chromatography (silica gel, 100-200 mesh) using 0-10% EtOAc in pet ether as eluent to afford 4- bromo-2-(methylthio)pyrimidine (6g, 83%) as off-white solid. LCMS: [M+H]+ 204.9.
References:
ONTARIO INSTITUTE FOR CANCER RESEARCH (OICR);AL-AWAR, Rima;ZEPEDA-VELAZQUEZ, Carlos Armando;PODA, Gennady;ISAAC, Methvin;UEHLING, David;WILSON, Brian;JOSEPH, Babu;LIU, Yong;SUBRAMANIAN, Pandiaraju;MAMAI, Ahmed;PRAKESCH, Michael;STILLE, Julia Kathleen WO2017/147700, 2017, A1 Location in patent:Paragraph 001094

49844-90-8
251 suppliers
$5.00/1g

959236-97-6
105 suppliers
$14.00/250mg