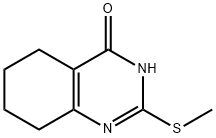
2-(Methylthio)-5,6,7,8-tetrahydroquinazolin-4-ol synthesis
- Product Name:2-(Methylthio)-5,6,7,8-tetrahydroquinazolin-4-ol
- CAS Number:34170-21-3
- Molecular formula:C9H12N2OS
- Molecular Weight:196.27
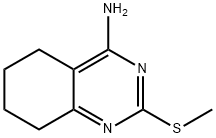
77766-03-1

34170-21-3
2-Methylthioalkyl-5,6,7,8-tetrahydroquinazolin-4-ylamine (5.09 g, 23.145 mmol) was used as a starting material and was mixed with 3-methyl-1-nitrosooxybutane (11.6 mL, 86.79 mmol) and trifluoroacetic acid (23.2 mL, 300.89 mmol) in chloroform (300 mL) to form a light yellow solution. The reaction system was heated to 60 °C and stirred continuously for 2 hours. Upon completion of the reaction, the reaction mixture was diluted with dichloromethane and subsequently extracted with 10% K2CO3 solution. After separation of the aqueous phase, the mixture was back-extracted once with dichloromethane, saturated with NaCl and then extracted with ethyl acetate. All organic phases were combined, dried with Na2SO4, filtered and the solvent evaporated. The resulting crude product was adsorbed onto an adsorbent and purified by chromatography on a Combiflash Companion XL system equipped with a SI50 silica gel column. The product grades were collected, and after evaporation of the solvent, the crystalline residue was ground in ether, filtered by pumping and dried under vacuum to yield 2.0 g of 5,6,7,8-tetrahydro-2-(methylthio)-4-quinazolinone in 57% yield as a crystal form.

77766-03-1
1 suppliers
inquiry

34170-21-3
50 suppliers
$58.00/100mg
Yield:34170-21-3 64%
Reaction Conditions:
with isopentyl nitrite in chloroform;trifluoroacetic acid; for 3 h;Heating;
References:
Sekiya, Tetsuo;Hiranuma, Hidetoshi;Uchide, Masayuki;Hata, Shunsuke;Yamada, Shun-Ichi [Chemical and pharmaceutical bulletin,1981,vol. 29,# 4,p. 948 - 954]
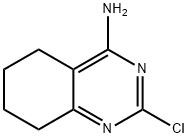
1127-86-2
1 suppliers
inquiry

34170-21-3
50 suppliers
$58.00/100mg
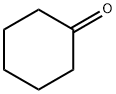
108-94-1
585 suppliers
$12.00/50g

34170-21-3
50 suppliers
$58.00/100mg
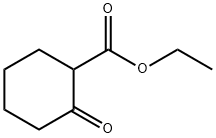
1655-07-8
310 suppliers
$6.00/5g

34170-21-3
50 suppliers
$58.00/100mg
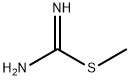
2986-19-8
70 suppliers
inquiry

1655-07-8
310 suppliers
$6.00/5g

34170-21-3
50 suppliers
$58.00/100mg