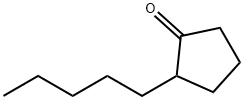
2-N-PENTYLCYCLOPENTANONE synthesis
- Product Name:2-N-PENTYLCYCLOPENTANONE
- CAS Number:4819-67-4
- Molecular formula:C10H18O
- Molecular Weight:154.25
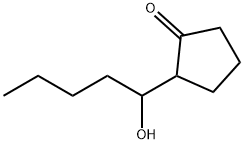
42558-01-0
5 suppliers
inquiry

4819-67-4
85 suppliers
$37.27/100G
Yield: 98%
Reaction Conditions:
with H3PO4/C;hydrogen;5%-palladium/activated carbon at 140; under 757.576 Torr; for 20 h;
Steps:
1
A 200 mL three-necked separable flask (made of glass) equipped with a dehydration tube was charged with 93.5 g (0.51 mol) of 2-(1-hydroxypentyl)-cyclopentanone (purity: 91%) obtained in Synthesis Example 1, 1.93 g of a phosphoric acid-supporting activated carbon (H3PO4/C; available from Taihei Chemical Industrial Co., Ltd.; powder, 22.1% hydrous product; phosphorus content: 0.012 (obtained by elemental analysis)), and 7.42 g (dry weight: 4.35 g) of 5% Pd/C (available from Evonik Degussa Japan Co., Ltd.; powder, 58.6% hydrous product). The contents of the flask were heated to 140°C while mixing under a pressure of 101 kPa (absolute pressure) while flowing a hydrogen gas (blowing the hydrogen gas into the reaction solution at a flow rate of 12 N-mL/h per 1 g of a pure content of 2-(1-hydroxypentyl)-cyclopentanone as the raw material). After reaching 140°C, the reaction solution was reacted for 20 h. After completion of the reaction, the final reaction product was subjected to quantitative determination by GC. As a result, it was confirmed that 0.50 mol of 2-pentyl-2-cyclopentanone was produced with a yield of 98%. Meanwhile, no 2-pentylcyclopentanol as a carbonyl group-reduced by-product was detected.
References:
Kao Corporation EP2487150, 2012, A1 Location in patent:Page/Page column 10
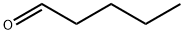
110-62-3
265 suppliers
$13.44/25ML

120-92-3
427 suppliers
$11.00/25g

4819-67-4
85 suppliers
$37.27/100G
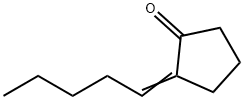
16424-35-4
14 suppliers
inquiry

4819-67-4
85 suppliers
$37.27/100G
25564-22-1
63 suppliers
$99.00/25mL

4819-67-4
85 suppliers
$37.27/100G

109-67-1
186 suppliers
$35.00/25 g

120-92-3
427 suppliers
$11.00/25g

4819-67-4
85 suppliers
$37.27/100G