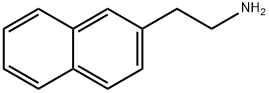
2-NAPHTHALEN-2-YL-ETHYLAMINE synthesis
- Product Name:2-NAPHTHALEN-2-YL-ETHYLAMINE
- CAS Number:2017-68-7
- Molecular formula:C12H13N
- Molecular Weight:171.24
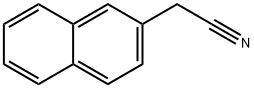
7498-57-9
165 suppliers
$6.00/1g

2017-68-7
55 suppliers
$45.00/25mg
Yield:2017-68-7 67%
Reaction Conditions:
with aluminium trichloride in water
Steps:
2.A 2-naphthaleneethanamine
Step A 2-naphthaleneethanamine Aluminum trichloride (7.6 g, 57 mmole) was added in portions to 30 mL of ether cooled to 0° C. under nitrogen. In a separate flask, lithium aluminum hydride (2.2 g, 57 mmole) was slurried in 30 mL of ether and cooled to -5° C. with an ice/acetone bath. The etherial aluminum trichloride solution was added dropwise to the LiAlH4 slurry at such a rate as to maintain a temperature of 0° C. Fifteen minutes after addition was complete, 2-naphthylacetonitrile (4.0 g, 24 mmole) in 40 mL of ether was added dropwise while maintaining a temperature of 0° C. The reaction mixture was stirred for an additional 15 minutes at 0° C. and then allowed to warm to room temperature for 1 hour. After recooling to 0° C., the reaction was quenched by careful dropwise addition of water. The mixture was diluted with ether, partitioned, and the aqueous phase was basified with 30% NH4 OH solution. Extraction of the aqueous phase with ether, combination of the organic phases, drying, and concentration gave a colorless oil (2.7g, 67% yield). 1 H MNR (CDCl3): δ 7.80 (m, 3H), 7.65 (s, 1H), 7.45 (m, 2H), 7.30 (m, 1H), 3.06 (t, 2H), 2.91 (t, 2H), 1.35 (s, 2H).
References:
E. I. Du Pont de Nemours and Company US5378708, 1995, A
1485-07-0
108 suppliers
$13.00/250mg

2017-68-7
55 suppliers
$45.00/25mg
![Carbamic acid, N-[2-(2-naphthalenyl)ethyl]-, ethyl ester](/CAS/20210305/GIF/73130-31-1.gif)
73130-31-1
0 suppliers
inquiry

2017-68-7
55 suppliers
$45.00/25mg
939-26-4
308 suppliers
$5.00/1g

2017-68-7
55 suppliers
$45.00/25mg