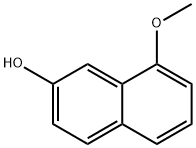
2-Naphthalenol,8-methoxy-(9CI) synthesis
- Product Name:2-Naphthalenol,8-methoxy-(9CI)
- CAS Number:91344-50-2
- Molecular formula:C11H10O2
- Molecular Weight:174.2
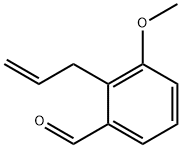
94956-98-6
44 suppliers
inquiry

91344-50-2
9 suppliers
inquiry
Yield:91344-50-2 80%
Reaction Conditions:
Stage #1: 2-allyl-3-methoxybenzaldehydewith oxygen;copper dichloride;palladium dichloride in tetrahydrofuran;methanol at 20; for 10 h;
Stage #2: with sodium hydroxide in tetrahydrofuran;methanol at 20; for 5 h;
Steps:
4.2. A representative synthetic procedure of skeleton 3, 4 or 7
General procedure: A representative synthetic procedure of skeleton 3, 4 or 7 is as follows: PdCl2 (10 mg, 5.6 mol %) and CuCl2 (200 mg, 1.5 mmol) were added to a solution of skeleton 2 or 5 (1.0 mmol) in the co-solvent of THF and methanol (20 mL, v/v=9:1) at rt. Then oxygen was bubbled into the mixture for 2 h, and stirred at rt for 8 h. NaOH (160 mg, 4.0 mmol) was added to a reaction mixture at rt. The reaction mixture was stirred at rt for 5 h. The residue was diluted with water (2 mL) and the mixture was extracted with EtOAc (3×20 mL). The combined organic layers were washed with brine, dried, filtered, and evaporated to afford crude product. Purification on silica gel (hexanes/EtOAc=10:1 to 6:1) afforded skeleton 3, 4 or 7.
References:
Chang, Meng-Yang;Chan, Chieh-Kai;Lin, Shin-Ying [Tetrahedron,2013,vol. 69,# 5,p. 1532 - 1538]
5309-18-2
51 suppliers
$73.00/10g

91344-50-2
9 suppliers
inquiry
575-38-2
227 suppliers
$6.00/1g

91344-50-2
9 suppliers
inquiry
![1-Naphthalenol, 7-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-](/CAS/20210305/GIF/220601-47-8.gif)
220601-47-8
0 suppliers
inquiry

91344-50-2
9 suppliers
inquiry
