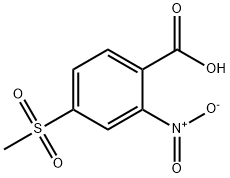
2-Nitro-4-methylsulfonylbenzoic acid synthesis
- Product Name:2-Nitro-4-methylsulfonylbenzoic acid
- CAS Number:110964-79-9
- Molecular formula:C8H7NO6S
- Molecular Weight:245.21
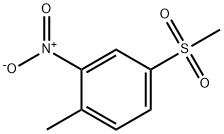
1671-49-4

110964-79-9
The general procedure for the synthesis of 2-nitro-4-methylsulfonylbenzoic acid from 2-nitro-4-methylsulfonyltoluene was as follows: 600.00 g of sulfuric acid with a mass fraction of 70% was added to a 1000 mL glass reactor, and a self-priming mixing device was activated. Subsequently, 97.83 g of 2-nitro-4-methylsulfonyltoluene with 99% purity and 1.95 g of vanadium pentoxide powder with 98% purity were added to the reactor and stirred continuously for 10 minutes. A condensation receiver with exhaust gas absorption was installed. The reaction system was rapidly heated to 140°C, and 230 g of nitric acid with a mass fraction of 68% was slowly added, while the oxygen feed rate was controlled to be 0.1 g/min, and the acceleration rate of the nitric acid droplet was 0.383 g/min, and the droplet addition was completed in about 10 hours. After completion of the dropwise addition, the reaction temperature is maintained at 140°C and stirring is continued until the mass fraction of unreacted 2-nitro-4-methylsulfonyltoluene is reduced to 1% or less (about 1 hour from the end of the dropwise addition to the completion of the reaction). The reaction solution was slowly cooled to 10-20°C with stirring and the product was separated by filtration. The filter cake was washed three times with 150 g of water and dried to give 110.20 g of 2-nitro-4-methylsulfonylbenzoic acid in 98.10% purity and 98.0% yield. 205 g of nitric acid with a mass fraction of 60% was recovered, which could be reused after evaporation and concentration. The small amount of nitrogen oxide exhaust gas generated during the reaction process was treated by air oxidation and absorption through an absorption device equipped with a 32% mass concentration of sodium hydroxide solution.
3185-99-7
284 suppliers
$5.00/10g

110964-79-9
286 suppliers
$5.00/5g
Yield:110964-79-9 98%
Reaction Conditions:
Stage #1:Methyl p-tolyl sulfone with nitric acid in dichloromethane at 10;Large scale;
Stage #2: with vanadium(V) oxide;sulfuric acid at 140;Large scale;
Steps:
1-4 Example 1
A method for preparing 2-nitro-4-methylsulfonylbenzoic acid, the preparation method comprising the following steps: In the nitrification reactor, 1.0 ton of dichloromethane was added, 0.8 ton of p-methylsulfone toluene was added, and the mixture was cooled to 10 ° C. The concentrated nitric acid was added dropwise, and the sample was analyzed by hollow sampling. After the nitration reaction was completed, it was placed in an oxidation reactor. Heating, distilling off dichloromethane, then adding 70% sulfuric acid as solvent, adding catalyst vanadium pentoxide, heating to 140 ° C, adding 60% nitric acid for oxidation reaction, after the reaction is finished, cooling to 25 ° C, centrifugation, mother liquor Applying to the oxidation reaction, the crude product is put into the reaction tank again, water is added, and the alkali solution is added to adjust the alkaline pH to 11. After filtering the impurities, the mother droplet is diluted with sulfuric acid to adjust the pH to 1.9, cooled and centrifuged, and dried to obtain a fine product
References:
Jiangsu Xin Xinlong Pharmaceutical Technology Co., Ltd.;Zhou Lidong;Zhou Jialin;Zhang Lan;Jiang Nan;Zhang Qin;Ji Zugen CN109503437, 2019, A Location in patent:Paragraph 0020-0033

1671-49-4
182 suppliers
$29.19/10g

64-19-7
1628 suppliers
$10.00/25ML

110964-79-9
286 suppliers
$5.00/5g

1671-49-4
182 suppliers
$29.19/10g

110964-79-9
286 suppliers
$5.00/5g
453-72-5
106 suppliers
$10.00/1g
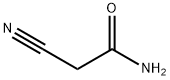
107-91-5
577 suppliers
$5.00/10g

110964-79-9
286 suppliers
$5.00/5g
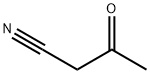
2469-99-0
92 suppliers
$16.00/1g
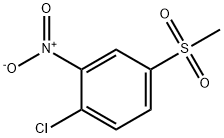
97-07-4
138 suppliers
$5.00/250mg

110964-79-9
286 suppliers
$5.00/5g