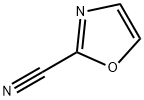
2-OXAZOLECARBONITRILE synthesis
- Product Name:2-OXAZOLECARBONITRILE
- CAS Number:68776-60-3
- Molecular formula:C4H2N2O
- Molecular Weight:94.07
884539-45-1

68776-60-3
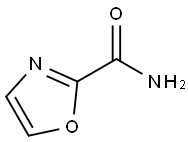
Example 112: Synthesis of 1,3-oxazole-2-carbonitrile; 1,3-oxazole-2-carboxamide (0.98 g, 8.8 mmol, 1.0 eq.) was dissolved in pyridine (17 mL), followed by the addition of phosphorus triclosan (1.2 mL, 12.2 mmol, 1.4 eq.). The reaction mixture was stirred at room temperature for 5 h. The solution was observed to change from a beige slurry to a brown color. After completion of the reaction, the mixture was diluted with ice water and extracted with ether. The aqueous layer was adjusted to pH 3 with 6 M hydrochloric acid and extracted again with ether. The combined ether extracts were washed sequentially with water and saturated brine, dried over anhydrous magnesium sulfate, filtered and concentrated under reduced pressure to give 1,3-oxazole-2-carbonitrile as an amber colored oil (0.61 g, 74% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 8.608 (s, 1H), 7.67 (s, 1H).

884539-45-1
43 suppliers
$60.00/100mg

68776-60-3
52 suppliers
inquiry
Yield:68776-60-3 74%
Reaction Conditions:
with pyridine;trichlorophosphate at 20; for 5 h;
Steps:
112
Example 112: 1,3-Oxazole-2-carbonitrile.; 0. prCN1 ,3-Oxazole-2-carboxamide (0.98 g, 8.8 mmol. 1.0 equiv) was dissolved in pyridine (17 mL) and treated with POCI3 (1.2 mL, 12.2 mmol, 1.4 equiv). The resultant beige slurry, which gave a brown solution, was stirred for 5 hours at room temperature. The reaction mixture was diluted with ice and the aqueous layer, which was adjusted to pH 3 with 6M HCI, was extracted with ether. The ether extract was washed with water, brine, dried over magnesium sulfate, filtered, concentrated in vacuo and gave the product as an amber oil (0.61 g, 74% yield): 1H NMR (400 MHz, DMSO-d6) δ 8.608 (s, 1 H), 7.67 (s, 1H).
References:
WO2006/43145,2006,A1 Location in patent:Page/Page column 131