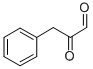
2-oxo-3-phenyl-propanal synthesis
- Product Name:2-oxo-3-phenyl-propanal
- CAS Number:56485-04-2
- Molecular formula:C9H8O2
- Molecular Weight:148.16
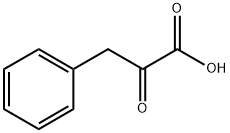
156-06-9
190 suppliers
$45.00/1g

56485-04-2
8 suppliers
inquiry
Yield:56485-04-2 80%
Reaction Conditions:
Stage #1: 2-oxo-3-(phenyl)propionic acidwith pyridine;acetic anhydride at 20; for 15 h;
Stage #2: with hydrogenchloride in dichloromethane;water;
Steps:
11.I
2-Oxo-3-phenyl-propionaldehyde. Phenylpyruvic acid (25.0 g, 152.0 mmol) was coevaporated twice with dry pyridine and then redissolved in dry pyridine (250 mL). To this solution was added acetic anhydride (170 mL, 1.8 moles) and the solution was stirred at ambient temperature for 15 h. The reaction progress was monitored by TLC. When the reaction was complete, the solution was evaporated to a viscous syrup. The syrup was dissolved in dichloromethane (700 mL) and then washed three times with 0.1 M aqueous HCl solution (3×200 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and evaporated to an amber-colored syrup. This product was purified by flash chromatography on silica gel (250 g) using dichloromethane as mobile phase. Appropriate fractions were pooled and evaporated to afford 24 g of a dry solid. This material was dissolved in THF (150 mL) and the solution was cooled in an ice-water bath. To the solution was added dropwise oxalyl chloride (51 mL, 580 mmol). After 10 min DMF (7.5 mL) was added to the reaction mixture and the reaction was stirred for 4 h at 0° C. Toluene (100 mL) was added and the reaction mixture was evaporated to give a thick oil. This material was coevaporated twice with toluene and the crude product was dried under vacuum for 5 h. The dried product was dissolved in a 1:1 mixture of THF-dichloromethane (200 mL) and the solution was cooled to -78° C. (dry ice-isopropanol bath) under argon. Then, lithium tri-tert-butoxyaluminohydride (152 mL of a 1.0 M solution in THF, 152 mmol) was added at a rate such that the internal temperature of the reaction was below -60° C. After addition was complete, the reaction was stirred below -60° C. for 10 h. The reaction was quenched by the slow addition of 2 M aqueous HCl solution (100 mL) and the mixture was allowed to warm to ambient temperature. The reaction mixture was diluted with dichloromethane (500 mL) and then washed twice with 0.1 M aqueous HCl solution (2×100 mL). The organic phase was dried over anhydrous sodium sulfate, filtered and evaporated to give an amber-colored syrup. This material was purified by flash chromatography on silica gel (250 g) starting with 95:5 heptane-ethyl acetate, then with 9:1 heptane-ethyl acetate as mobile phase. Appropriate fractions were pooled and evaporated to afford 13.5 g (80%) of the desired compound.
References:
US7692022,2010,B2 Location in patent:Page/Page column 45-46

77252-94-9
1 suppliers
inquiry

56485-04-2
8 suppliers
inquiry
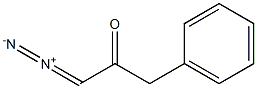
4250-02-6
4 suppliers
inquiry

56485-04-2
8 suppliers
inquiry
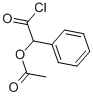
1638-63-7
60 suppliers
$45.00/50mg

56485-04-2
8 suppliers
inquiry
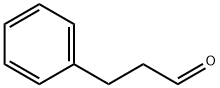
104-53-0
303 suppliers
$6.00/5g

56485-04-2
8 suppliers
inquiry