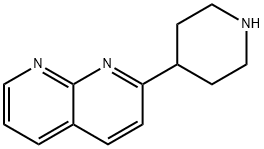
2-(piperidin-4-yl)-1,8-naphthyridine synthesis
- Product Name:2-(piperidin-4-yl)-1,8-naphthyridine
- CAS Number:870089-65-9
- Molecular formula:C13H15N3
- Molecular Weight:213.28
Yield:-
Reaction Conditions:
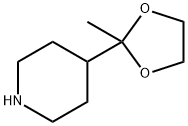
Stage #1:4-(2-methyl-[1,3]dioxolan-2-yl)-piperidine with hydrogenchloride in ethanol;water at 0; for 24 h;Heating / reflux;
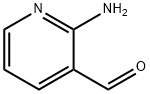
Stage #2:2-Aminonicotinaldehyde with sodium carbonate in ethanol;water
Stage #3: with potassium carbonate in ethanol;water at 20 - 100; for 26 h;
Steps:
12 Synthesis of 2-piperidin-4-yl-[1,8]naphthyridine
Synthesis of 2-piperidin-4-yl-[1,8]naphthyridine 51 g (300 mmoles) of 4-(2-methyl-[1,3]dioxolan-2-yl)-piperidine (prepared according to J. Org. Chem.; 29; 1964; 2898-2903) is solubilized in 550 ml of ethanol. This mixture is cooled down to 0° C. using an ice bath. 85 ml of 6N hydrochloric acid (510 mmoles) is added. The mixture is heated under reflux of ethanol for 24 hours. Then 20.4 g (510 mmoles) of soda in pellets is added in order to neutralize the pH then 32.13 g (260 mmoles) of 2-aminopyridine-3-carboxyaldehyde and 200 ml of ethanol are added. Finally 40.8 g (295 mmoles) of potassium carbonate is added. This mixture is stirred at 100° C. for 8 hours then for 18 hours at ambient temperature. The ethanol is evaporated off under reduced pressure, then diluted with water and with butanol. The 2 phases are separated and the product is thus extracted with butanol. The organic phase is dried over sodium sulphate then filtered and evaporated under reduced pressure. A brown solid is recovered, 65 g of which is used without purification for the following. TLC: Rf=0.1 (silica gel, eluent: CH2Cl2/MeOH/NH4OH 89:10:1 1H-NMR (MeOD): δ from 1.90 to 2.10 (m, 4H, and '); from 2.8 to 2.90 (m, 2H, or '); from 3.12 to 3.28 (m, 3H, + or); from 7.58 to 7.68 (m, 2H, and); from 8.38 to 8.45 (m, 2H, and); 9.03 (m, 1H,). MS: 270 (MH+)
References:
Proskelia SAS US2008/58348, 2008, A1 Location in patent:Page/Page column 20