
2-(piperidin-4-yl)-3-(trifluoromethyl)pyridine synthesis
- Product Name:2-(piperidin-4-yl)-3-(trifluoromethyl)pyridine
- CAS Number:914220-27-2
- Molecular formula:C11H13F3N2
- Molecular Weight:230.23
![1-Piperidinecarboxylic acid, 4-[3-(trifluoromethyl)-2-pyridinyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/946399-74-2.gif)
946399-74-2
0 suppliers
inquiry

914220-27-2
2 suppliers
inquiry
Yield:914220-27-2 93%
Reaction Conditions:
Stage #1: tert-butyl 4-(3-(trifluoromethyl)pyridin-2-yl)piperidine-1-carboxylatewith trifluoroacetic acid in dichloromethane at 0 - 20; for 3 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane; pH=8 - 9;
Steps:
28.3
Into a 50-mL round-bottom flask, was placed a solution of tert-butyl 4-(3- (trifluoromethyl)pyridin-2-yl)piperidine-l-carboxylate (379.8 mg, 1.10 mmol, 1.00 equiv, 96%) in dichloromethane (10 mL). This was followed by the addition of trifluoroacetic acid (5 mL) dropwise with stirring at 0°C. The resulting solution was stirred for 3 h at room temperature. The pH value of the solution was adjusted to 8- 9 with sat sodium bicarbonate. The resulting solution was extracted with 6x50 mL of dichloromethane and the organic layers combined and dried over anhydrous sodium sulfate. The solids were filtered out. The resulting mixture was concentrated under vacuum. This resulted in 237.4 mg (93%) of 2-(piperidin-4-yl)-3- (trifluoromethyl)pyridine as yellow oil.LC-MS: (ES, m/z): 231 [M+H]+
References:
WO2011/28947,2011,A2 Location in patent:Page/Page column 101; 102-103
![[2,4'-Bipyridine]-1'(2'H)-carboxylic acid, 3',6'-dihydro-3-(trifluoromethyl)-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/683242-50-4.gif)
683242-50-4
0 suppliers
inquiry

914220-27-2
2 suppliers
inquiry
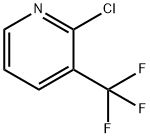
65753-47-1
377 suppliers
$8.00/5g

914220-27-2
2 suppliers
inquiry