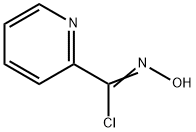
2-PyridinecarboxiMidoyl chloride, N-hydroxy- synthesis
- Product Name:2-PyridinecarboxiMidoyl chloride, N-hydroxy-
- CAS Number:69716-28-5
- Molecular formula:C6H5ClN2O
- Molecular Weight:156.57
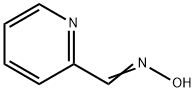
873-69-8

69716-28-5
2-Pyridine carboxaldehyde oxime (288a, 180 mg, 1.47 mmol) was used as raw material and dissolved in DMF (2 mL) at 20 °C. Subsequently, N-chlorosuccinimide (NCS, 216 mg, 1.62 mmol) was added all at once. The reaction mixture was stirred at 20 °C for 12 hours. After completion of the reaction, the mixture was concentrated. The residue was poured into water (20 mL) and the aqueous phase was extracted with ethyl acetate (50 mL × 3). The combined organic phases were washed with brine (20 mL × 2), dried over anhydrous Na2SO4, filtered and concentrated in vacuo to afford 2-pyridine carboxaldehyde oxime chloride (288b, 200 mg, yield: 87.1%) as a brown solid.1H NMR (400 MHz, CDCl3) δ 10.79 (br s, 1H), 8.72 (d, J = 4.2 Hz, 1H) , 7.94 (d, J = 8.2 Hz, 1H), 7.80 (dt, J = 1.7, 7.8 Hz, 1H), 7.39 (ddd, J = 0.8, 5.0, 7.4 Hz, 1H), 2.79 (s, 1H).
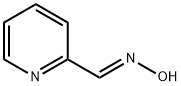
1193-96-0
57 suppliers
$19.00/5g

69716-28-5
30 suppliers
inquiry
Yield: 75%
Reaction Conditions:
with N-chloro-succinimide in N,N-dimethyl-formamide at 20 - 60;
Steps:
Preparation of Int-IV-A: (E,Z)-N-Hydroxypicolinimidoyl chloride[00184] To a colorless, homogeneous solution of commercially available (E)- picolinaldehyde oxime (6.75 g, 55.3 mmol) in N,N-dimethylformamide (55 mL) at room temperature was added N-chlorosuccinimide (7.38 g, 55.3 mmol) portion-wise. After the addition of ~ 1/5 of the NCS, the reaction mixture was immersed in an oil bath at 60 0C, and the remaining NCS was added portion-wise over 1.5 h. After the addition was complete, the homogeneous reaction mixture was stirred for 60 min. at 60 0C and was then cooled to room temperature. Water (400 mL) was added, and the aqueous mixture was extracted with ether (3 x 200 mL). The organic layer was collected, washed with water (2 x 200 mL), washed with a saturated aqueous solution of brine (100 mL), and dried over anhydrous magnesium sulfate. Concentration under reduced pressure afforded (E,Z)-N-hydroxypicolinimidoyl chloride (6.45 g, 41.2 mmol, 75% yield) as a tan solid. The compound had an HPLC retention time = 0.515 min.-Column: CHROMOLITH SpeedROD 4.6 x 50 mm (4 min.); Solvent A = 10% MeOH, 90% H2O, 0.1% TFA;Solvent B = 90% MeOH, 10% H2O, 0.1% TFA. LC/MS M+l = 156.8. 1H NMR (400 MHz, CDCl3) δ ppm 7.37-7.43 (m, IH), 7.80 (td, J=7.78, 1.76 Hz, IH), 7.91-7.97 (m, IH), 8.72 (d, J=4.02 Hz, IH), and 9.85 (br. s., IH).
References:
BRISTOL-MYERS SQUIBB COMPANY;GILMORE, John L.;SHEPPECK, James E. WO2011/17578, 2011, A1 Location in patent:Page/Page column 80

873-69-8
195 suppliers
$8.00/5g

69716-28-5
30 suppliers
inquiry
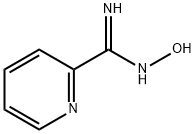
1772-01-6
139 suppliers
$10.00/1g

69716-28-5
30 suppliers
inquiry
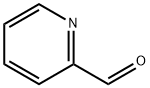
1121-60-4
524 suppliers
$10.60/10gm:

69716-28-5
30 suppliers
inquiry