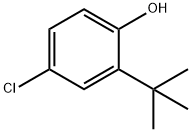
2-tert-Butyl-4-chlorophenol synthesis
- Product Name:2-tert-Butyl-4-chlorophenol
- CAS Number:13395-85-2
- Molecular formula:C10H13ClO
- Molecular Weight:184.66
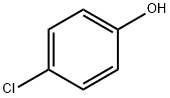
106-48-9

75-65-0

13395-85-2
Step 1: Synthesis of 2-(tert-butyl)-4-chlorophenol (P19a) Concentrated hydrochloric acid was added to a mixture of 4-chlorophenol (50 g, 0.39 mol) and tert-butanol (57.6 g, 0.78 mol). Subsequently, sulfuric acid (40 mL) was added and the reaction mixture was stirred at room temperature for 48 hours. After completion of the reaction, the mixture was poured into ice water and extracted twice with ethyl acetate (EA). The organic layers were combined, washed sequentially with water (3 times) and brine, and dried over anhydrous sodium sulfate (Na2SO4). After filtration, the filtrate was concentrated and purified by column chromatography (eluent: petroleum ether/ethyl acetate = 40/1) to afford compound P19a (48.5 g, 68% yield) as a colorless solid.

106-48-9
497 suppliers
$10.00/5 g

75-65-0
784 suppliers
$10.00/10ml

13395-85-2
59 suppliers
$30.00/25mg
Yield: 71%
Reaction Conditions:
with sulfuric acid for 48 h;Inert atmosphere;
Steps:
1 Salicylaldehyde Synthesis.
The ligand precursor 3-ter -butyl-5-chlorosalicylaldehyde has been previously reported, and FontWeight="Bold" FontSize="10" H NMR assignments are included that match well with those in the literature. The yield represents average isolated yields. The precursor to the salicylaldehyde, 2-tert- butyl-4-chlorophenol, was synthesized according to literature procedure. 4-Chlorophenol (Combi-B locks, 8.00 g, 62.2 mmol) was dissolved in tert-butyl alcohol (Aldrich, 1 1.9 mL, 124 mmol), and 7.50 mL of concentrated H2S04 was added dropwise over 5 minutes, turning the solution from a pale yellow to a light orange. The solution was stirred for 2 days, neutralized with Na2C03 (aq), and then extracted into diethyl ether and dried over Na2S04. The product was concentrated and purified by column chromatography (95:5, hex:EtOAc) resulting in a yellow oil (71% isolated yield). Using a modified Duff reaction, 2-/ert-butyl-4- chlorophenol was formylated as reported by Jacobsen et al. The product was purified by column chromatography (90: 10, hex:EtOAc) to yield a crystalline yellow solid (24% isolated yield). NMR spectrum in ppm (CDC13, 400 MHz): δ 1 1.72 (s, I H); 9.82 (s, IH); 7.46 (d, J = 2.6 Hz, IH); 7.38 (d, J = 2.6 Hz, IH); 1.41 (s, 12H).
References:
CORNELL UNIVERSITY;COATES, Geoffrey;WHITEHEAD, Julie WO2016/25675, 2016, A1 Location in patent:Paragraph 0100
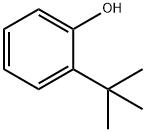
88-18-6
184 suppliers
$16.00/25mL

13395-85-2
59 suppliers
$30.00/25mg
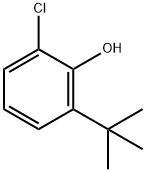
4237-37-0
19 suppliers
inquiry

88-18-6
184 suppliers
$16.00/25mL

13395-85-2
59 suppliers
$30.00/25mg

88-18-6
184 suppliers
$16.00/25mL

13395-85-2
59 suppliers
$30.00/25mg

4237-37-0
19 suppliers
inquiry

13395-86-3
2 suppliers
inquiry