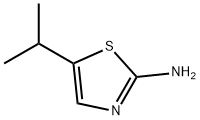
2-Thiazolamine, 5-(1-methylethyl)- synthesis
- Product Name:2-Thiazolamine, 5-(1-methylethyl)-
- CAS Number:101080-15-3
- Molecular formula:C6H10N2S
- Molecular Weight:142.22

64932-36-1
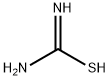
17356-08-0

101080-15-3
General procedure: To a dichloromethane/dioxane (V/V = 4/1) solution of isovaleraldehyde (1.721 g, 20 mmol) was slowly added dropwise a dichloromethane/dioxane (V/V = 4/1) solution of bromine (1.0 mL, 20 mmol). The reaction mixture was stirred at 10 °C to give 2-bromoisovaleraldehyde intermediate. Subsequently, 2-bromoisovaleraldehyde intermediate was slowly added dropwise to a dichloromethane/dioxane (30 mL) solution of thiourea (1.523 g, 20 mmol). Ethanol (6 mL) and triethylamine (2.424 g, 24 mmol) were added and the reaction was carried out at room temperature for 20 hours. Upon completion of the reaction, 100 mL of distilled water was added to the reaction mixture and the pH was adjusted to 12 with 12 N sodium hydroxide solution. after continued stirring for 1 h at room temperature, extraction was carried out with dichloromethane and water. The organic layer was washed with saturated brine and dried with anhydrous magnesium sulfate. Finally, the target compound 5-isopropylthiazol-2-amine was purified by silica gel column chromatography to give the target compound 5-isopropylthiazol-2-amine (1.202 g, 42.26% yield).

7664-41-7
519 suppliers
$15.00/100ml
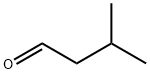
590-86-3
379 suppliers
$10.00/5g

101080-15-3
85 suppliers
$53.00/25 mg
Yield: 42%
Reaction Conditions:
with bromine;thiourea in 1,4-dioxane;ethanol;dichloromethane
Steps:
4 Preparation of a Compound of Formula (II): 2-amino-5-isopropyl-1,3-thiazole
EXAMPLE 4 Preparation of a Compound of Formula (II): 2-amino-5-isopropyl-1,3-thiazole 3-Methylbutanaldehyde (2 ml; 18.6 mmol) was dissolved in 15 ml of dioxane. A solution 2% v/v of bromine in dioxane (47.81 ml; 18.6 mmol) was dropped therein at 0° C. The mixture was maintained at room temperature under stirring for 2 hours, then 2.83 g (37.2 mmol) of thiourea and 10 ml of ethanol were added. After 6 hours at room temperature the solution was evaporated to dryness, the residue was dissolved in methylene chloride and the product extracted with 1M hydrochloric acid; the aqueous layer was made basic by using 30% ammonium hydrate and extracted again with methylene chloride. The organic phase was dried over sodium sulfate and evaporated under vacuum. The residue was chromatographed on a silica gel column, eluding with cyclohexane-ethylacetate to give 1.1 g (42% yield) of the title compound. 1H-NMR (DMSO-d6) δppm: 6.6 (s, 2H, NH2); 6.58 (s, 1H, thiazole CH); 2.9 (m, 1H, CHMe2); 1.18 (s, 3H, MeCHMe); 1.17 (s, 3H, MeCHMe).
References:
Pevarello, Paolo;Amici, Raffaella;Traquandi, Gabriella;Villa, Manuela;Vulpetti, Anna;Isacchi, Antonella US2003/187040, 2003, A1

17356-08-0
2 suppliers
inquiry

590-86-3
379 suppliers
$10.00/5g

101080-15-3
85 suppliers
$53.00/25 mg

64932-36-1
11 suppliers
inquiry

17356-08-0
2 suppliers
inquiry

101080-15-3
85 suppliers
$53.00/25 mg
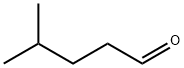
1119-16-0
77 suppliers
$133.00/100mg

17356-08-0
2 suppliers
inquiry

101080-15-3
85 suppliers
$53.00/25 mg

590-86-3
379 suppliers
$10.00/5g

101080-15-3
85 suppliers
$53.00/25 mg