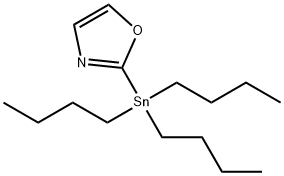
2-(TRI-N-BUTYLSTANNYL)OXAZOLE synthesis
- Product Name:2-(TRI-N-BUTYLSTANNYL)OXAZOLE
- CAS Number:145214-05-7
- Molecular formula:C15H29NOSn
- Molecular Weight:358.11
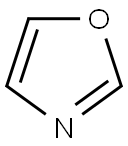
288-42-6
226 suppliers
$15.00/1g
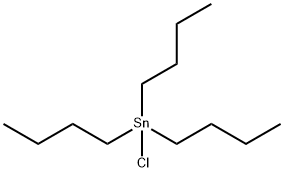
1461-22-9
218 suppliers
$10.00/10g

145214-05-7
87 suppliers
$63.20/250mg
Yield:145214-05-7 77%
Reaction Conditions:
Stage #1:oxazol with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Stage #2:tributyltin chloride in tetrahydrofuran;hexaneInert atmosphere;
Steps:
xix.a 2-(Tributylstannyl)oxazole I39
To a solution of oxazole (500 mg, 7.25 mmol) in THF (15 mL) at -78 °C under N2 was added n-BuLi (2.5 M solution in hexanes, 2.9 mL, 7.32 mmol) dropwise and the mixture was stirred at -78 °C for 30 min. Tributylchlorostannane (1.96 mL, 7.25 mmol) was then added and the mixture was allowed to warm to room temperature and stirred for 1 h. The solvent was removed under reduced pressure and residue was taken up in hexanes (50 mL). The resulting precipitate was removed by filtration and the filtrate was concentrated under reduced pressure to give the title compound I39 (2.0 g, 77%) as colourless oil. 1H NMR (400 MHz, DMSO-de) d 8.20 (s, 1H), 7.20 (s, 1H), 1.59 - 1.49 (m, 6H), 1.31 - 1.26 (m, 6H), 1.16 - 1.10 (m, 6H), 0.83 (t, J = 7.3 Hz, 9H).
References:
CTXT PTY LIMITED;STUPPLE, Paul, Anthony;LAGIAKOS, Helen, Rachel;FOITZIK, Richard, Charles;CAMERINO, Michelle, Ang;NIKOLAKOPOULOS, George;BOZIKIS, Ylva, Elisabet, Bergman;KERSTEN, Wilhelmus, Johannes, Antonius;WALKER, Scott, Raymond;HUBERT, Jonathan, Grant WO2020/2587, 2020, A1 Location in patent:Page/Page column 72-73

288-42-6
226 suppliers
$15.00/1g

145214-05-7
87 suppliers
$63.20/250mg