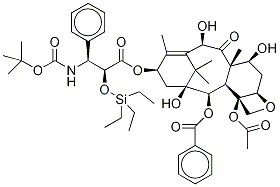
2'-Triethylsilyldocetaxel synthesis
- Product Name:2'-Triethylsilyldocetaxel
- CAS Number:162871-12-7
- Molecular formula:C49H67NO14Si
- Molecular Weight:922.14
![Benzenepropanoic acid, β-[[(1,1-dimethylethoxy)carbonyl]amino]-α-[(triethylsilyl)oxy]-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-4,6-bis[[(2,2,2-trichloroethoxy)carbonyl]oxy]-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (αR,βS)-](/CAS/20240320/GIF/152089-14-0.gif)
152089-14-0
0 suppliers
inquiry

162871-12-7
12 suppliers
inquiry
Yield:162871-12-7 97%
Reaction Conditions:
with ammonium chloride;zinc in methanol at 20; for 1 h;Time;
Steps:
5
At room temperature add 50 ml of methanol, 8 g of active zinc powder and 8 g of NH4C1 into a 100 ml reaction containet Stirring evenly, add 5 g of 2’-TES-7, lO-TROCdocetaxel. Stir 1 hr, TLC shows reaction completion, filter, evaporate to obtain white solid 3.0 g of 2’-TES-docetaxel (yield 97%).10184] The examples 1-5 show that the yield when deprotecting TROC according to the invention (example 2-5) is improved compared to current technology (example 1). Example 1 is a prior art-type acetic acid deprotection, and as well as suffering from worse yield, also has more steps thaninventive method.
References:
US2014/371474,2014,A1 Location in patent:Paragraph 0183