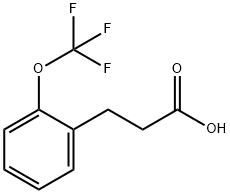
2-(TrifluoroMethoxy)-benzenepropanoic acid synthesis
- Product Name:2-(TrifluoroMethoxy)-benzenepropanoic acid
- CAS Number:914636-53-6
- Molecular formula:C10H9F3O3
- Molecular Weight:234.17
![2-Propenoic acid, 3-[2-(trifluoromethoxy)phenyl]-, methyl ester, (2E)-](/CAS/20180906/GIF/1202578-19-5.gif)
1202578-19-5
0 suppliers
inquiry

914636-53-6
18 suppliers
$52.00/250mg
Yield:914636-53-6 76%
Reaction Conditions:
Stage #1: methyl (2E)-3-[2-(trifluoromethoxy)phenyl]prop-2-enoatewith hydrogen;palladium 10% on activated carbon in methanol at 20;Inert atmosphere;
Stage #2: with water;sodium hydroxide in methanol at 20;
Stage #3: with hydrogenchloride in methanol;water;
Steps:
63.B
(63B) 3-[2-(Trifluoromethoxy)phenyl]propionic acidTo a methanol solution of methyl (2E)-3-[2-(trifluoromethoxy)phenyl]acrylate (12.2 g, 49.6 mmol) produced in (63A), 10% palladium carbon (1.10 g) was added under a nitrogen atmosphere, and the resulting mixture was stirred under a hydrogen atmosphere at room temperature for 1.5 hours. After the reaction system was replaced with nitrogen, dichloromethane (90 mL) was added thereto, and the resulting mixture was filtered through a Celite filter. Then, the solvent was distilled off under reduced pressure, whereby a crude product was obtained. This crude product was used in the subsequent reaction step without performing further purification procedures.The obtained crude product was dissolved in methanol (80 mL), and a 2 N aqueous solution of sodium hydroxide (42 mL, 84 mmol) was added thereto at room temperature, and then, the resulting mixture was stirred at room temperature for 30 minutes. After water was added to the reaction solution, 2 N hydrochloric acid (42 mL, 84 mmol) was added thereto, and the organic matter was extracted with dichloromethane. The organic layer was washed sequentially with water and a saturated sodium chloride solution, then dried over anhydrous sodium sulfate and filtered. Then, the solvent was distilled off under reduced pressure, whereby the objective title compound was obtained as a white solid (8.86 g, yield: 76%).1H NMR (CDCl3, 400 MHz): δ2.69 (2H, t, J=7.7 Hz), 3.03 (2H, t, J=7.7 Hz), 7.20-7.33 (4H, m)
References:
US2011/53974,2011,A1 Location in patent:Page/Page column 80
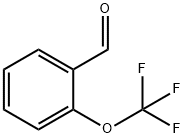
94651-33-9
216 suppliers
$10.00/1g

914636-53-6
18 suppliers
$52.00/250mg