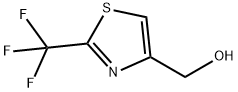
[2-(Trifluoromethyl)-1,3-thiazol-4-yl]methanol synthesis
- Product Name:[2-(Trifluoromethyl)-1,3-thiazol-4-yl]methanol
- CAS Number:133046-47-6
- Molecular formula:C5H4F3NOS
- Molecular Weight:183.15
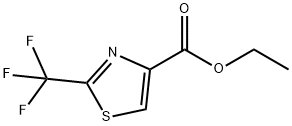
133046-46-5
87 suppliers
$60.00/100mg
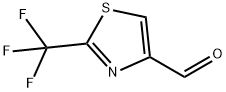
133046-48-7
45 suppliers
$364.00/250mg
![[2-(Trifluoromethyl)-1,3-thiazol-4-yl]methanol](/CAS/GIF/133046-47-6.gif)
133046-47-6
32 suppliers
$65.00/10mg
Yield:133046-48-7 43% ,133046-47-6 19%
Reaction Conditions:
with diisobutylaluminium hydride in dichloromethane at -78; for 1 h;
Steps:
41
Diisobutyl aluminium hydride (IM in dichloromethane, 16.7 ml, 16.7 mmol) was added dropwise to a solution of Description 26 (1.88 g, 8.37 mmol) in anhydrous dichloromethane (10 ml) at -78 0C. The reaction was stirred at -78 0C for lhr, and then excess aq. 2Ν hydrochloric acid was added and the reaction allowed to warm to room temperature. The reaction was extracted into ethyl acetate (x 5) and the organic layer washed with water and saturated aqueous NH4Cl, then dried (MgSO4) and evaporated.Purification by flash column chromatography on silica (eluent: 25% ethyl acetate in hexane) gave the title compound as a pale yellow solid (650 mg, 43%) 1H NMR (400 MHz, DMSO) δ 10.01 (1 H, s), 9.07 (1 H, s). (Additionally, some of the corresponding alcohol (19 %) was isolated).
References:
WO2006/122200,2006,A1 Location in patent:Page/Page column 47

133046-46-5
87 suppliers
$60.00/100mg
![[2-(Trifluoromethyl)-1,3-thiazol-4-yl]methanol](/CAS/GIF/133046-47-6.gif)
133046-47-6
32 suppliers
$65.00/10mg