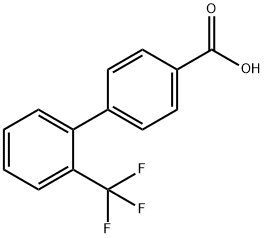
2'-TRIFLUOROMETHYLBIPHENYL-4-CARBOXYLIC ACID synthesis
- Product Name:2'-TRIFLUOROMETHYLBIPHENYL-4-CARBOXYLIC ACID
- CAS Number:198205-79-7
- Molecular formula:C14H9F3O2
- Molecular Weight:266.22
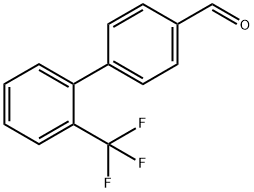
198205-95-7

198205-79-7
General procedure for the synthesis of 2'-trifluoromethyl diphenyl-4-carboxylic acid from 2'-(trifluoromethyl)-[1,1'-biphenyl]-4-carboxaldehyde: The product of step A (0.4 g, 1.6 mmol) was refluxed for 2 h under stirring with a suspension formed by potassium permanganate (KMnO4, 0.502 g, 3.2 mmol) in dioxane (15 mL). After completion of the reaction, the mixture was filtered through a silica gel column and the filter cake was washed with ethyl acetate (EtOAc, 30 mL). Subsequently, the solvent in the filtrate was evaporated to dryness to afford the target product 2'-trifluoromethyl diphenyl-4-carboxylic acid (0.35 g, 81.6% yield) as a white solid. The 1H-NMR (CDCl3) data of the product were as follows: δ 7.30-7.58 (m, 5H); 7.76 (d, 1H, J = 8.15 Hz); 8.11 (d, 2H, J = 8.03 Hz).
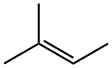
513-35-9
187 suppliers
$12.00/25g

198205-95-7
76 suppliers
$10.00/250mg

198205-79-7
77 suppliers
$35.00/100mg
Yield: 80%
Reaction Conditions:
with sodium chlorite;sodium dihydrogenphosphate in water;tert-butyl alcohol at 20; for 5.5 h;
Steps:
18 Compound 18a: 2'-(Trifluoromethyl)-[1,1'-biphenyl]-4-carboxylic acid
To a solution of 2'-(trifluoromethyl)-[1,1'-biphenyl]-4-carboxaldehyde (0.147 g, 0.59 mol) in t-BuOH (9 mL) and 2-METHYL-2-BUTENE (9 mL) was added a solution of NACIO2 (0.496, 5.50 mmol) AND NAH2P04 (0.588 g, 4.9 mmol) in water (6 mL) in four portions over 0.5 h. The resulting reaction mixture was stirred for 5 h at room temperature, concentrated in vacuo, and the residue was diluted with water. The aqueous phase was extracted with CH2CI2 (3 x). The product in the combined organic phases was then extracted into 1 N NAOH (3 x). The CH2C12 layer was discarded, the combined aqueous layers were acidified with 1 N HCl, and the product was back extracted with CH2CL2 (3 x). The combined organic phases were then dried over NA2S04, filtered, and concentrated in vacuo to provide the title compound (0.125 g, 80%) as a white solid. The crude material was of sufficient purity (>90%) to be used in subsequent STEPS.'H-NMR (CD30D); 8 8.06 (D, J=8. 0 Hz, 2H), 7.79 (D,@ J=7. 6 Hz, 1H), 7.66 (t, J=7. 6 Hz, 1H), 7.57 (t, J=7. 6 Hz, 1H), 7. 42-7. 36 (overlapping d at 7.41 and d at 7.37, J=8. 0 Hz for both d, 3H).
References:
ASTRAZENECA AB WO2004/60882, 2004, A1 Location in patent:Page 56-57

198205-95-7
76 suppliers
$10.00/250mg

198205-79-7
77 suppliers
$35.00/100mg