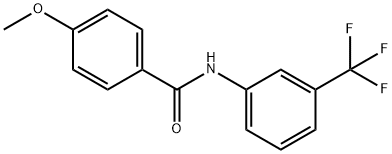
N-[3-(TrifluoroMethyl)phenyl]-4-MethoxybenzaMide, 97% synthesis
- Product Name:N-[3-(TrifluoroMethyl)phenyl]-4-MethoxybenzaMide, 97%
- CAS Number:200630-42-8
- Molecular formula:C15H12F3NO2
- Molecular Weight:295.26
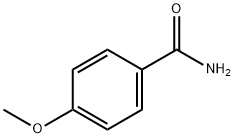
3424-93-9
197 suppliers
$14.00/1g
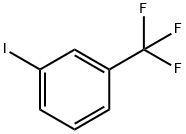
401-81-0
238 suppliers
$10.00/10g
![N-[3-(TrifluoroMethyl)phenyl]-4-MethoxybenzaMide, 97%](/CAS/20150408/GIF/200630-42-8.gif)
200630-42-8
6 suppliers
$28.60/25MG
Yield:200630-42-8 44%
Reaction Conditions:
with copper(I) oxide;potassium phosphate;tetra-n-propylammonium bromide in water at 130; for 24 h;
Steps:
General procedure for N-arylation of amides
The N-nucleophile (2.21 mmol), Cu2O (Sigma-Aldrich, 99.99% purity, 0.147-0.294 mmol), K3PO4(2.94 mmol), the aryl halide (1.47 mmol), phase transfer catalyst (0.147-0.294 mmol) and water(0.40 mL) were added to a reaction vial and a screw cap was fitted to it. The reaction mixture wasstirred under air in a closed system at 130°C for 24 h, then the heterogeneous mixture was cooledto RT and diluted with dichloromethane. The resulting solution was directly filtered through apad of Celite. The combined organic extracts were dried with anhydrous Na2SO4 and the solventwas removed under reduced pressure. The crude product was purified by silica-gel columnchromatography to afford the N-arylated product. The identity and purity of all products wasconfirmed by 1H and 13C NMR spectroscopic analysis.
References:
Yong, Fui-Fong;Teo, Yong-Chua;Chua, Guan-Leong;Lim, Gina Shiyun;Lin, Yizhen [Tetrahedron Letters,2011,vol. 52,# 11,p. 1169 - 1172] Location in patent:supporting information; experimental part