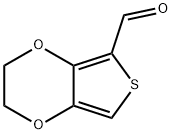
2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXINE-5-CARBALDEHYDE synthesis
- Product Name:2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXINE-5-CARBALDEHYDE
- CAS Number:204905-77-1
- Molecular formula:C7H6O3S
- Molecular Weight:170.19
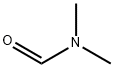
68-12-2
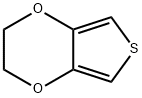
126213-50-1
![2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXINE-5-CARBALDEHYDE](/CAS/GIF/204905-77-1.gif)
204905-77-1
General procedure for the synthesis of 2-(3,4-ethenyldioxythiophene) formaldehyde from N,N-dimethylformamide (DMF) and 3,4-ethenyldioxythiophene (EDOT): to a mixture containing EDOT (15.0 g, 0.1 mol) and DMF (11.0 g, 0.15 mol) was added slowly and dropwise at 0 °C a mixture of 2-(3,4-ethenyldioxythiophene) and 3,4-ethenyldioxythiophene (EDOT) dissolved in anhydrous dichloromethane ( 80 mL) in triphosgene (BTC, 11.9 g, 0.04 mol). The reaction mixture was warmed to 35 °C and stirred continuously for 1 h. Subsequently, it was cooled to room temperature and poured into ice water (250 mL). The pH of the aqueous phase was adjusted to 7-8 with 10% sodium hydroxide solution and the organic phase was separated. The aqueous phase was extracted three times with dichloromethane. All organic phases were combined and dried with anhydrous magnesium sulfate. After removal of the solvent by distillation under reduced pressure, the crude product was recrystallized by 95% ethanol to give a white needle-like crystal product (15.5 g, 86.3% yield). The product was characterized by 1H NMR (400 MHz): δ/ppm: 9.90 (s, 1H), 6.79 (s, 1H), 4.38-4.25 (m, 4H).
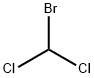
75-27-4
138 suppliers
$28.30/1mL

126213-50-1
455 suppliers
$8.00/5g
![2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXINE-5,7-DICARBALDEHYDE](/CAS/GIF/211235-87-9.gif)
211235-87-9
40 suppliers
$45.00/100mg
![2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXINE-5-CARBALDEHYDE](/CAS/GIF/204905-77-1.gif)
204905-77-1
120 suppliers
$13.00/250mg
Yield:204905-77-1 22% ,211235-87-9 43%
Reaction Conditions:
Stage #1: bromodichloromethane;3,4-(ethylenedioxy)thiophenewith 1,1'-bis-(diphenylphosphino)ferrocene;potassium phosphate;palladium diacetate;acetic anhydride;silver carbonate in acetonitrile at 60; for 48 h;Schlenk technique;Inert atmosphere;
Stage #2: with hydrogenchloride in dichloromethane at 30;Overall yield = 66 %;
Steps:
General Procedure
General procedure: To a 50 mL of Schlenk tube were added 1 or 3 (0.2 mmol, 1.0 equiv), Pd(OAc)2 (10 mol%), dppf (10 mol%) under air, followed by K3PO4·3H2O (0.3 mmol, 1.5 equiv) and Ag2CO3 (0.3 mmol, 1.5 equiv) . The mixture was then evacuated and back filled with N2 (3 times). Bromodichoromethane (0.4 mmol, 2.0 equiv), Ac2O (2 mmol, 190 uL) and CH3CN (1 mL) were added subsequently. The Schlenk tube was screw capped and put into a preheated oil bath (60 °C). After stirring for 24 hours, the reaction mixture was cooled to room temperature, diluted with CH2Cl2 and Ethyl Acetate, then filtered with a pad of silica gel. The isolated yield was given by a hydrolysis pathway, in which the concentrated reaction mixture was diluted with 5 mL CH2Cl2 and 10 mL 3 N HCl and stirred over night. The reaction mixture was extracted with dichloromethane (3 times) and the solvent was removed under rotary evaporation. The residue was then purified by a preparative TLC to give product 2 or 4.
References:
Bao, Yan;Wang, Jian-Yong;Zhang, Ya-Xuan;Li, Yan;Wang, Xi-Sheng [Tetrahedron Letters,2018,vol. 59,# 32,p. 3147 - 3150] Location in patent:supporting information

126213-50-1
455 suppliers
$8.00/5g
![2,3-DIHYDROTHIENO[3,4-B][1,4]DIOXINE-5-CARBALDEHYDE](/CAS/GIF/204905-77-1.gif)
204905-77-1
120 suppliers
$13.00/250mg