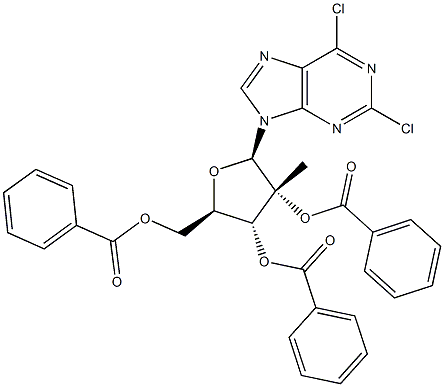
2,6-Dichloro-9-(2-C-Methyl-2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)purine synthesis
- Product Name:2,6-Dichloro-9-(2-C-Methyl-2,3,5-tri-O-benzoyl-β-D-ribofuranosyl)purine
- CAS Number:205171-10-4
- Molecular formula:C32H24Cl2N4O7
- Molecular Weight:647.46
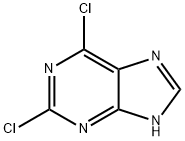
5451-40-1
506 suppliers
$10.00/1g
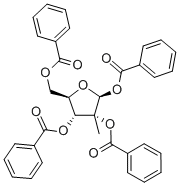
15397-15-6
144 suppliers
$18.00/1g

205171-10-4
19 suppliers
$1066.00/1g
Yield:205171-10-4 97%
Reaction Conditions:
with trimethylsilyl trifluoromethanesulfonate;1,8-diazabicyclo[5.4.0]undec-7-ene in acetonitrile at 0 - 65; for 5.33 h;
Steps:
73.a
Step a: To β-D-ribofuranose, 2-C-methyl-, 1,2,3,5-tetrabenzoate (4.0 g, 6.89 mmol, 1 equiv.) and 2,6-dichloropurine (1.43 g, 7.58 mmol, 1.1 equiv.) in acetonitrile (23 mL) at 0 °C was added 1,8-Diazabicyclo[5.4.0]undec-7-ene (2.58 mL, 17.23 mmol, 2.5 equiv.) followed by trimethylsilyl trifluoromethanesulfonate (5.11 mL, 28.25 mmol, 4.1 equiv.) dropwise over 5 minutes. The reaction mixture was stirred at 0 °C for 15 minutes and heated at 65 °C for 5 hours. After cooling to room temperature the reaction was diluted with dichloromethane, washed with sat. aq. sodium bicarbonate (x2), and brine (x1). The organics were dried over MgSO4, filtered and concentrated under reduced pressure. The desired product was obtained following column chromatography (SiO2, 25% to 66% EtOAc/Hexane) as a white solid (1.30 g, 97%).
References:
WO2017/120508,2017,A1 Location in patent:Paragraph 0289

15397-15-6
144 suppliers
$18.00/1g

5451-40-1
506 suppliers
$10.00/1g

205171-10-4
19 suppliers
$1066.00/1g
98-88-4
598 suppliers
$10.00/5g

205171-10-4
19 suppliers
$1066.00/1g
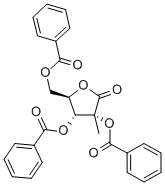
7392-74-7
77 suppliers
$50.00/2g

205171-10-4
19 suppliers
$1066.00/1g
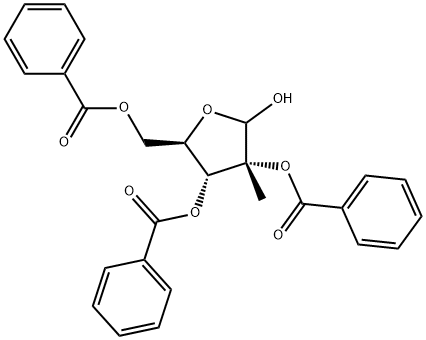
30361-17-2
24 suppliers
inquiry

205171-10-4
19 suppliers
$1066.00/1g