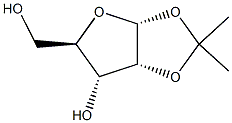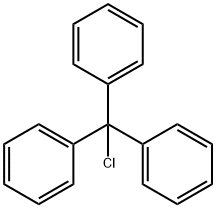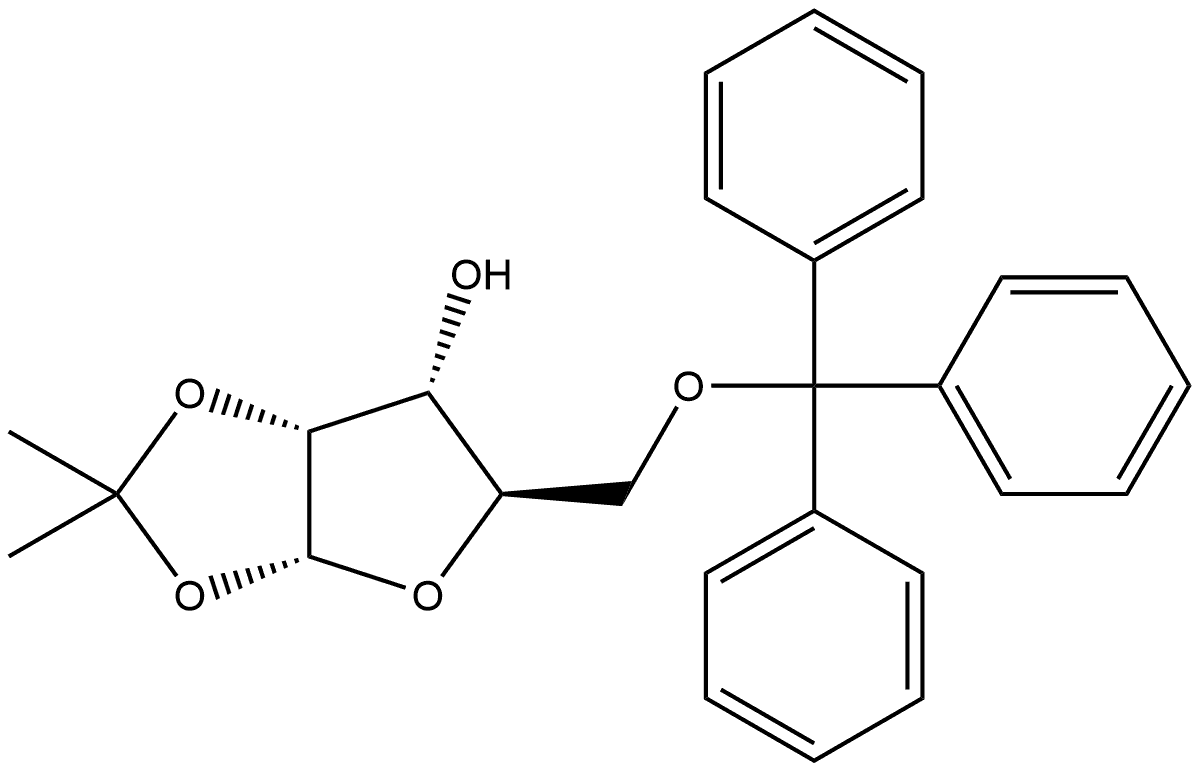
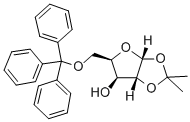
1,2-O-Isopropylidene-5-O-(triphenylmethyl)-alpha-D-ribofuranose synthesis
- Product Name:1,2-O-Isopropylidene-5-O-(triphenylmethyl)-alpha-D-ribofuranose
- CAS Number:20590-57-2
- Molecular formula:C27H28O5
- Molecular Weight:432.51
20590-54-9
2 suppliers
inquiry

20590-57-2
3 suppliers
inquiry
Yield:20590-57-2 99%
Reaction Conditions:
with sodium tetrahydroborate in ethanol;dichloromethane at 0 - 20; for 0.5 h;Inert atmosphere;
Steps:
b 4.1.1 1,2-O-Isopropylidene-5-O-trityl-α-d-ribofuranose (12)
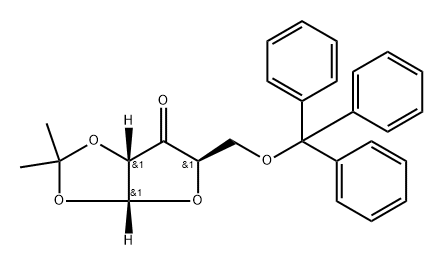
IBX was added to a solution of 11 [12b] (10.8g, 0.025mol) in MeCN (230mL) and the resulting mixture was heated at reflux for 5h. After cooling to room temperature, the solid parts were filtered off, and the filtrate was concentrated in vacuo. Chromatography of the residue on silica gel (n-hexane/ethyl acetate, 5:1) gave 10.0g (93%) of 1,2-O-isopropylidene-5-O-trityl-α-d-erythro-pentofuranos-3-ulose [13] as a white amorphous solid; [α]D26+123.5 (c 0.20, CHCl3), lit [13]. [α]D+132 (c 4.7, CHCl3, temperature at 21-22°C). IR (neat) υmax 1010, 1077, 1152, 1215, 1375, 1448, 1489, 1773cm-1; 1H NMR (600MHz, CDCl3): δ 1.47 (s, 6H, 2×CH3), 3.32 (dd, 1H, J=2.6Hz, J=10.1Hz, H-5), 3.50 (dd, 1H, J=2.4Hz, J=10.1Hz, H-5), 4.41 (m, 1H, H-4), 4.54 (dd, 1H, J=1.0Hz, J=4.5Hz, H-2), 6.33 (d, 1H, J=4.5Hz, H-1), 7.23-7.25 (m, 3H, Ph), 7.28-7.31 (m, 6H, Ph), 7.34-7.36 (m, 6H, Ph); 13C NMR (100MHz, CDCl3): δ 27.2 (CH3), 27.7 (CH3), 64.4 (C-5), 76.9 (C-2), 80.0 (C-4), 87.4 (Cq), 103.6 (C-1), 114.2 (Cq), 127.2 (3×CHPh), 127.9 (6×CHPh), 128.6 (6×CHPh), 143.2 (3×Ci), 210.2 (C-3). Anal. Calcd for C27H26O5: C, 75.33; H, 6.09. Found: C, 75.20, H, 6.18. To a solution of the obtained ulose [13] (9.9g, 0.023mol) in a mixture of EtOH/CH2Cl2 (378mL, 8:1) that had been pre-cooled to 0°C was added NaBH4 (1.74g, 0.046mol). After stirring at 0°C for 10min and another 20minat room temperature, the solvents were evaporated, and the residue was partitioned between CH2Cl2 (290mL) and brine (340mL). The separated aqueous layer was then washed with CH2Cl2 (2×290mL). The combined organic extracts were dried over Na2SO4, the solvent was evaporated, and the residue was subjected to flash chromatography on silica gel (n-hexane/ethyl acetate, 5:1) to afford 9.85g (99%) of compound 12 as a white foam; [α]D26+25.5 (c 0.20, CHCl3); lit [13]. [α]D+25.8 (c 4.5, CHCl3, temperature at 21-22°C). IR (neat) υmax 1011, 1064, 1114, 1163, 1213, 1373, 1447, 1490cm-1; 1H NMR (400MHz, CDCl3): δ 1.38 (s, 3H, CH3), 1.57 (s, 3H, CH3), 2.31 (d, 1H, J=9.6Hz, OH), 3.27 (dd, 1H, J=4.8Hz, J=10.4Hz, H-5), 3.41 (dd, 1H, J=3.0Hz, J=10.4Hz, H-5), 3.88-4.00 (m, 2H, H-3, H-4), 4.57-4.59 (m, 1H, H-2), 5.88 (d, 1H, J=3.8Hz, H-1), 7.21-7.26 (m, 3H, Ph), 7.27-7.32 (m, 6H, Ph), 7.44-7.48 (m, 6H, Ph); 13C NMR (100MHz, CDCl3): δ 26.5 (CH3), 26.6 (CH3), 63.0 (C-5), 72.3 (C-3), 78.5(C-2), 79.8 (C-4), 86.7 (Cq), 104.2 (C-1), 112.6 (Cq), 127.0 (3×CHPh), 127.8 (6×CHPh), 128.7 (6×CHPh), 143.8 (3×Ci). Anal. Calcd for C27H28O5: C, 74.98; H, 6.53. Found: C, 74.82; H, 6.64.
References:
Jacková, Dominika;Martinková, Miroslava;Gonda, Jozef;Stanková, Kvetoslava;Bago Pilátová, Martina;Herich, Peter;Ko?í?ek, Jozef [Carbohydrate Research,2017,vol. 451,p. 59 - 71]
20590-53-8
8 suppliers
inquiry

20590-57-2
3 suppliers
inquiry