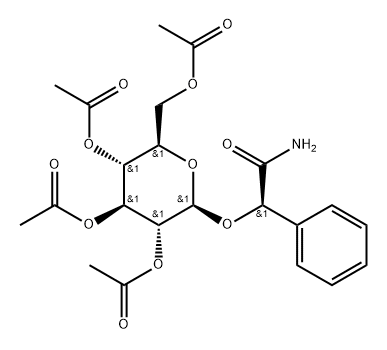
(αR)-α-[(2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl)oxy]benzeneacetamide synthesis
- Product Name:(αR)-α-[(2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl)oxy]benzeneacetamide
- CAS Number:207512-68-3
- Molecular formula:C22H27NO11
- Molecular Weight:481.45

108-24-7
0 suppliers
$14.00/250ML
151649-54-6
0 suppliers
inquiry
![(αR)-α-[(2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl)oxy]benzeneacetamide](/CAS/20211123/GIF/207512-68-3.gif)
207512-68-3
9 suppliers
$120.00/25mg
Yield:207512-68-3 32 mg
Reaction Conditions:
with pyridine at 20; for 3 h;
Steps:
(R)-2,3,4,6-tetra-O-acetyl-β-D-glucopyranosyloxy(phenyl)acetamide(6)
General procedure: Glucoside 16 (34 mg, 0.054 mmol) was dissolved in CH2Cl2 (0.5 mL) andMeOH (1 mL), and 1 M sodium methoxide (0.02 mL) was added. The mixturewas kept at rt for 2 h. The mixture was made neutral with Amberlite IR-120(H+). The resin was filtered off and thoroughly washed with MeOH, and thecombined filtrates were concentrated. The residue was dissolved in Py (1 mL),and acetic anhydride (0.2 mL) was added. The reaction mixture was stirred atrt for 3 h and coevaporated with toluene. The residue was purified by silica gelcolumn chromatography (2:1 toluene-acetone) to provide 18 (26 mg, quant.):amorphous powder; [α]D +14 (c 1.1, CHCl3); δH (600 MHz, CDCl3): 2.00, 2.01,2.02, and 2.09 (12 H, all s, 4×Ac), 3.61 (1 , m, H-5’), 3.95 (1 H, dd, J5,6a =2.1, J6a,6b = 12.3, H-6a’), 4.12 (1 H, dd, J5,6a = 5.3, H-6b’), 4.69 (1 H, d, J1,2 =7.9, H-1’), 5.05 (1 H, t, J3,4 = J4,5 = 9.7, H-4’), 5.12 (1 H, dd, J2,3 = 9.5, H-2’),5.13 (1 H, s, H-7), 5.23 (1 H, t, H-3’), 5.82 and 6.52 (2 H, both br.s, CONH2),7.31-7.49 (5 , m, Ph); δC (150 MHz, CDCl3): 20.5, 20.6, 20.65, and 20.7(4×Ac), 61.7 (C-6’), 68.1, 71.7, 72.0, and 72.5 (C-2’, 3’, 4’, 5’), 82.0 (C-7), 100.1(C-1’), 127.1-135.9 (Ph), 169.3, 169.6, 170.2 and 170.5 (4×Ac), 173.0 (C-8); HMRS-ESI (positive mode): m/z 504.1474 [M+Na]+; calcd. for C22H27NNaO11504.1476
References:
Yashunsky, Dmitry V.;Kulakovskaya, Ekaterina V.;Kulakovskaya, Tatiana V.;Zhukova, Olga S.;Kiselevskiy, Mikhail V.;Nifantiev, Nikolay E. [Journal of Carbohydrate Chemistry,2015,vol. 34,# 8,p. 460 - 474]