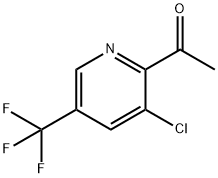
ETHANONE, 1-[3-CHLORO-5-(TRIFLUOROMETHYL)-2-PYRIDINYL]- synthesis
- Product Name:ETHANONE, 1-[3-CHLORO-5-(TRIFLUOROMETHYL)-2-PYRIDINYL]-
- CAS Number:207994-12-5
- Molecular formula:C8H5ClF3NO
- Molecular Weight:223.58
80194-70-3
128 suppliers
inquiry

75-16-1
274 suppliers
$12.00/10ml
![ETHANONE, 1-[3-CHLORO-5-(TRIFLUOROMETHYL)-2-PYRIDINYL]-](/CAS/GIF/207994-12-5.gif)
207994-12-5
38 suppliers
$60.00/100mg
Yield:207994-12-5 46%
Reaction Conditions:
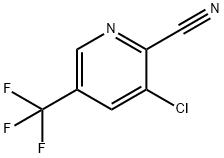
Stage #1: 3-chloro-2-cyano-5-(trifluoromethyl)pyridine;methylmagnesium bromide in tetrahydrofuran;toluene at -5 - 20; for 7 h;
Stage #2: with hydrogenchloride;water in tetrahydrofuran;toluene at 20; for 3 h;
Steps:
10
Example 10 : Preparation of 2-amino-1-[3-chloro-5-(trifluoromethyl)-2-pyridinyl]ethanol; Preparation of 1-[3-chloro-5-(trifluoromethyl)-2-pyridinyl] ethanone; To 225 ml of dry toluene were added 210 ml (0.29 mol) of a 1.4 M solution of methylmagnesium bromide in toluene/tetrahydrofuran 75:25. The solution was cooled to - 5°C and 30 g (0.145 mol) of 3-chloro-5-(trifluoromethyl)-2-pyridinecarbonitrile, were slowly added in 2 hours at 0°C. After addition, the dark solution was further stirred at room temperature for 5 hours. The reaction mixture was neutralized by 350 ml of 1N hydrochloric acid and stirred 3 hours at room temperature. The aqueous phase was then reextracted by ethyl acetate (3 x 200 ml), washed with water (300 ml) and dried over magnesium. The solvent was evaporated under reduced pressure to give 33.2 g of the crude product as a brown oil. The crude product was purified by flash chromatography on silica gel (eluent: heptane/ethyl acetate 9:1) to give 1-[3-chloro-5-(trifluoromethyl)-2-pyridinyl]ethanone: 13.1 g (40 %) as a yellow oil. Mass spectrum: [M+1] = 224.
References:
EP1548007,2005,A1 Location in patent:Page/Page column 44