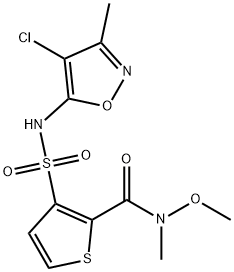
3-(N-(4-chloro-3-Methylisoxazol-5-yl)sulfaMoyl)-N-Methoxy-N-Methylthiophene-2-carboxaMide synthesis
- Product Name:3-(N-(4-chloro-3-Methylisoxazol-5-yl)sulfaMoyl)-N-Methoxy-N-Methylthiophene-2-carboxaMide
- CAS Number:210421-71-9
- Molecular formula:C11H12ClN3O5S2
- Molecular Weight:365.81

6638-79-5
592 suppliers
$6.00/25g
![2-Thiophenecarboxylicacid, 3-[[(4-chloro-3-Methyl-5-isoxazolyl)aMino]sulfonyl]-](/CAS/GIF/184040-74-2.gif)
184040-74-2
37 suppliers
$92.00/100mg

210421-71-9
19 suppliers
$505.38/5MG
Yield:210421-71-9 53%
Reaction Conditions:
Stage #1: 3{[(4-chloro-3-methyl-5-isoxazolyl)amino]sulfonyl}-2-thiophenecarboxylic acidwith 1,1'-carbonyldiimidazole in tetrahydrofuran at 20; for 0.5 h;
Stage #2: N,O-dimethylhydroxylamine*hydrochloridewith 1H-imidazole in tetrahydrofuran at 20; for 5 h;
Steps:
1-2 3-[(4-chloro-3-methyl-1,2-oxazol-5-yl)suliamoyl]-N-methoxy-N-methylthiophene-2-carboxamide
To a mixture of 3-[(4-chloro-3-methyl-1,2-oxazol-5-yl)sulfamoyl]thiophene-2-carboxylic acid (2.5 g, 7.8 mmol) described in Production Example 1-1 and tetrahydrofuran (25 mL) was added 1,1′-carbonyldiimidazole (2.0 g, 12 mmol) at room temperature, followed by stirring at that temperature for 30 minutes. Imidazole (1.1 g, 16 mmol) and N,O-dimethylhydroxylamine hydrochloride (1.2 g, 12 mmol) were added successively at room temperature to the reaction mixture, followed by stirring at that temperature for 5 hours. A 1 N aqueous solution of hydrochloric acid (50 mL) was added to the reaction mixture, which was then extracted with ethyl acetate. The organic layer was washed with brine and dried over anhydrous sodium sulfate, and the solvent was distilled away under reduced pressure. The residue was purified by silica gel column chromatography (chloroform:methanol=9:1) to give the title compound (1.5 g, 53% yield). 1H-NMR Spectrum (CDCl3) δ (ppm): 2.23 (3H, s), 3.45 (3H., s), 3.74 (3H, s), 7.47 (1H, d, J=5.3 Hz), 7.53 (1H, d, J=5.3 Hz).
References:
US2013/197045,2013,A1 Location in patent:Paragraph 0140; 0141; 0142
59337-92-7
235 suppliers
$10.00/5g

210421-71-9
19 suppliers
$505.38/5MG