
2,3-DiMethoxy-7-Methyl-6-(phenylMethyl)-1-propylnaphthalene synthesis
- Product Name:2,3-DiMethoxy-7-Methyl-6-(phenylMethyl)-1-propylnaphthalene
- CAS Number:213971-42-7
- Molecular formula:C23H26O2
- Molecular Weight:334.45
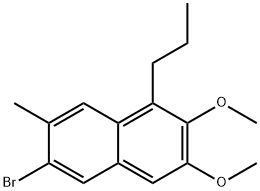
213971-39-2
13 suppliers
inquiry

213971-42-7
12 suppliers
inquiry
Yield:213971-42-7 88%
Reaction Conditions:
with hydrogen;benzaldehyde;palladium in ethanol;hexane;dichloromethane;
Steps:
Experimental Section
Compound 18b (800 mg, 2.47 mmol) in 25 mL of dry ether was cooled to 0° C. under nitrogen. n-Butyllithium (3.0 mmol) was added as a solution in hexane. The mixture was stirred for 15 min at 0° C., and then benzaldehyde (424 mg, 4 mmol) was added. The mixture was stirred at ambient temperature under nitrogen for 1 h. The reaction mixture was acidified, and the organic layer was separated, washed with water and brine, and was dried over magnesium sulfate. After filtration, the ether was evaporated to give a semi-solid which was dissolved in ethanol and hydrogenated on a Parr hydrogenator with 10% palladium on carbon and 60 psi hydrogen pressure at room temperature for 2 h. The reaction mixture was vacuum filtered through celite, and the celite was washed with ether. The solvent was evaporated, and the residual oil was purified by silica column chromatography using dichloromethane to give 727 mg (2.17 mmol, 88% yield) of 19d as a crystalline solid, mp 83-85° C. 1 H NMR:(ppm) 7.64 (s, 1H), 7.39 (s, 1H), 7.14-7.29 (m, 5H), 6.95 (s, 1H), 4.09 (s, 2H), 3.91 (s, 3H), 3.86 (s, 3H), 3.01 (t, 2H), 2.36 (s, 3H), 1.68 (m, 2H), 1.05 (t, 3H). Anal. (C23 H26 O2) C,H.
References:
US6124498,2000,A

213971-39-2
13 suppliers
inquiry

74-88-4
356 suppliers
$15.00/10g

213971-41-6
5 suppliers
inquiry

213971-42-7
12 suppliers
inquiry
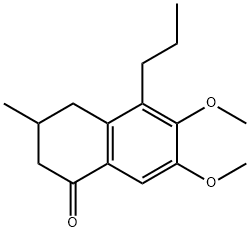
213971-37-0
5 suppliers
inquiry

213971-42-7
12 suppliers
inquiry
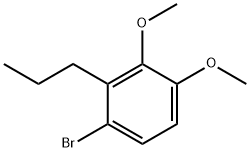
142601-49-8
16 suppliers
inquiry

213971-42-7
12 suppliers
inquiry

215171-91-8
0 suppliers
inquiry

213971-42-7
12 suppliers
inquiry