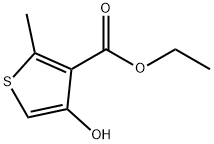
4-Hydroxy-2-methyl-3-thiophenecarboxylic acid ethyl ester synthesis
- Product Name:4-Hydroxy-2-methyl-3-thiophenecarboxylic acid ethyl ester
- CAS Number:2158-82-9
- Molecular formula:C8H10O3S
- Molecular Weight:186.23
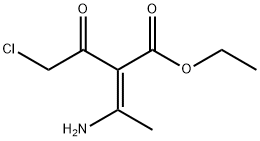
78120-39-5
8 suppliers
inquiry

2158-82-9
13 suppliers
inquiry
Yield:2158-82-9 76%
Reaction Conditions:
with sodium hydrogen sulfide monohydrate;water in ethanol at 20; for 1.5 h;
Steps:
16 Compound 103-3
Add (E)-3-amino-2-(2-chloroacetyl)-2-butenoic acid ethyl ester (103-2) (12.0g, 58.4mmol) to absolute ethanol (120mL) at room temperature At room temperature, an aqueous solution (24 mL) of sodium hydrosulfide monohydrate (16.8 g, 226.8 mmol) was slowly added dropwise.After the addition is complete, react at room temperature for 1.5 hours.After the reaction was completed, the solution was slowly added to water (340 mL), a yellow solid precipitated, stirred for 30 minutes, and filtered.The filter cake was collected, and the filter cake was dissolved with ethyl acetate (200 mL), washed with saturated brine (200 mL) and water (200 mL), respectively, dried with anhydrous sodium sulfate, and filtered.The filtrate was concentrated under reduced pressure to obtain ethyl 4-hydroxy-2-methylthiophene-3-carboxylate (103-3) (8.3 g, yield: 76%).
References:
CN113493438,2021,A Location in patent:Paragraph 0456; 0462-0465
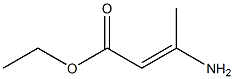
41867-20-3
10 suppliers
inquiry

2158-82-9
13 suppliers
inquiry