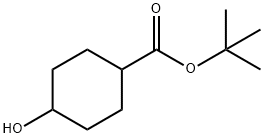
Cyclohexanecarboxylic acid, 4-hydroxy-, 1,1-dimethylethyl ester synthesis
- Product Name:Cyclohexanecarboxylic acid, 4-hydroxy-, 1,1-dimethylethyl ester
- CAS Number:220179-93-1
- Molecular formula:C11H20O3
- Molecular Weight:200.27
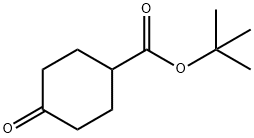
38446-95-6
77 suppliers
$65.00/50mg

220179-93-1
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with methanol;sodium tetrahydroborate at 0 - 10; for 1 h;
Steps:
Intermediate 8a: tert-Butyl 4-hydroxycyclohexanecarboxylate
Sodium borohydride (6ii mg, i6.0 mmol) was added slowly to a solution of tert-butyl4-oxocyclohexanecarboxylate E08495_830.1 (3.20 g, i6.0 mmol) in methanol (30 mL) at 0 °C. After stirred at iO °C for i hour, the mixture was added to water (50 mL), concentrated under reduced pressure and extracted with ethyl acetate (40 mL) twice. The combined organic layers were dried over Na2SO4(), filtered and concentrated toafford the crude product (3.20 g, crude) as colorless oil, which was used for the next step without further purification. ‘H N1.VIR (300 1VIHz, CDC13) 3.9i - 3.85 (m, 0.4H), 3.65 -3.57 (m, 0.6H), 2.30 -2.27 (m, 0.4H), 2.i8 - 2.iO (m, 0.6H), 2.04- i.89 (m, 3H), i.66 - i.60 (m, 3H), i.53 - i.35 (m, iOH), i.30 - i.2i (m, 2H).
References:
WO2019/1420,2019,A1 Location in patent:Page/Page column 98