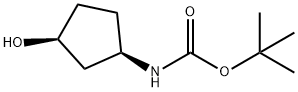
Carbamic acid, [(1R,3S)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI) synthesis
- Product Name:Carbamic acid, [(1R,3S)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)
- CAS Number:225641-84-9
- Molecular formula:C10H19NO3
- Molecular Weight:201.26
![Carbamic acid, [(1S,4R)-4-hydroxy-2-cyclopenten-1-yl]-, 1,1-dimethylethyl](/CAS/GIF/201054-55-9.gif)
201054-55-9
![Carbamic acid, [(1R,3S)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/225641-84-9.gif)
225641-84-9
General procedure for the synthesis of tert-butyl ((1R,3S)-3-hydroxycyclopentyl)carbamate from (1S,4R)-4-cyclopenten-1-ol-2-amino-tert-butyloxycarbonyl: Tert-butyl (1S,4R)-4-hydroxycyclopent-2-enylcarbamate (2.0 g, 10 mmol) was dissolved in methanol (20 mL), and the reaction flask was subjected to three times nitrogen Displacement. 10% Pd/C catalyst (0.2 g, 10%, w/w) was added to the above solution, followed by three displacements with hydrogen. The reaction mixture was stirred at room temperature and hydrogen atmosphere (1 atm) for 14 hours. Upon completion of the reaction, the catalyst was removed by diatomaceous earth filtration. The filtrate was concentrated to afford the target product ((1R,3S)-3-hydroxycyclopentyl)carbamic acid tert-butyl ester (1.9 g, yield: 95%).
![Carbamic acid, [(1S,4R)-4-hydroxy-2-cyclopenten-1-yl]-, 1,1-dimethylethyl](/CAS/GIF/201054-55-9.gif)
201054-55-9
29 suppliers
$65.00/10mg
![Carbamic acid, [(1R,3S)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/225641-84-9.gif)
225641-84-9
81 suppliers
$55.00/100mg
Yield:225641-84-9 95%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in methanol at 20; for 14 h;Inert atmosphere;
Steps:
B tert-butyl (1R,3S)-3-hydroxycyclopentylcarbamate
tert-butyl (1s, 4r)-4-hydroxycyclopent-2-enylcarbamate (2.0 g, 10 mmol) was dissolved in methanol (20 mL), the reaction flask was purged with nitrogen air three times. 10% Pd/C (0.2 g, 10%, w/w) was added to the above solution, and then replaced with hydrogen three times.
The reaction solution was stirred at room temperature under hydrogen atmosphere (1 atm) for 14 hours, and filtered through celite.
The filtrate was concentrated to give the title compound (1.9 g, yield: 95%).
References:
US2016/200730,2016,A1 Location in patent:Paragraph 0500; 0501
![Tert-Butyl 2-Oxa-3-Azabicyclo[2.2.1]Hept-5-Ene-3-Carboxylate](/CAS/GIF/CB73144508.gif)
99027-90-4
42 suppliers
$17.00/100mg
![Carbamic acid, [(1R,3S)-3-hydroxycyclopentyl]-, 1,1-dimethylethyl ester (9CI)](/CAS/GIF/225641-84-9.gif)
225641-84-9
81 suppliers
$55.00/100mg