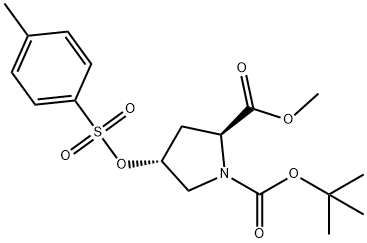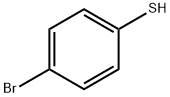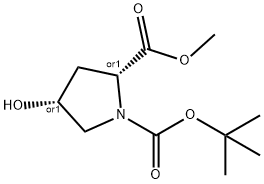
1,2-Pyrrolidinedicarboxylic acid, 4-hydroxy-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-rel- synthesis
- Product Name:1,2-Pyrrolidinedicarboxylic acid, 4-hydroxy-, 1-(1,1-dimethylethyl) 2-methyl ester, (2R,4R)-rel-
- CAS Number:227935-38-8
- Molecular formula:C11H19NO5
- Molecular Weight:245.27
Yield:227935-38-8 40%
Reaction Conditions:
Stage #1: para-bromobenzenethiolwith sodium ethanolate in ethanol at 20; for 0.5 h;
Stage #2: (2S,4R)-4-(toluene-4-sulfonyloxy)-pyrrolidine-1,2-dicarboxylic acid 1-tert-butyl ester 2-ethyl ester in ethanol; for 48 h;
Steps:
5
Sodium ethoxide (112MG, 1. 65mmol) was added slowly to a stirred solution of 4- bromothiophenol (302mg, 1. 65MMOL) in EtOH (6M .) AT, ROOM temperature under a nitrogen atmosphere. A solution of (2S, 4R)-4- (TOLUENE-4-SULFONYLOXY)-PYRROLIDINE-1, 2- DICARBOXYLIC acid 1-tert-butyl ester 2-methyl ester (CAS Reg. No. 88043-21-4) (300mg, 0. 75mmol) in 1ML ETOH was added after 30 minutes and the solution was stirred for 48h. The reaction mixture was poured into 0.5M NAOH (50MI) and extracted with CH2CI2 (2 x 50ml). The combined organics were dried (magnesium sulphate) and concentrated under vacuum. Flash column chromatography yielded the product as a pink solid (120mg, 40%). 'H-NMR (400MHZ, CDCl3) 5 = 1.25 (3H, t), 1.40 (9H, s), 2.00 (1 H, s), 2.60 (1 H, m), 3.35 (1H, m), 3.60 (1H, m), 3.90 (1H, s), 4.18 (2H, q), 4.22 (1H, m), 7.35 (2H, d), 7.40 (2H, d). LRMS (Electrospray) : m/z [MNA+] 454.
References:
WO2004/39367,2004,A1 Location in patent:Page/Page column 60-61