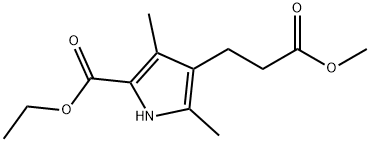
ethyl 4-(2-methoxycarbonylethyl)-3,5-dimethyl-1H-pyrrole-2-carboxylate synthesis
- Product Name:ethyl 4-(2-methoxycarbonylethyl)-3,5-dimethyl-1H-pyrrole-2-carboxylate
- CAS Number:2386-37-0
- Molecular formula:C13H19NO4
- Molecular Weight:253.29
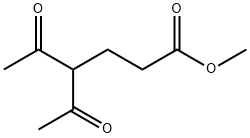
13984-53-7
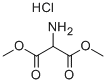
16115-80-3

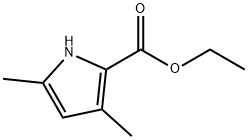
2386-37-0
b) Preparation of compound (5): In a 4.5 liter Keller round bottom flask equipped with a reflux condenser, thermometer, dropping funnel and argon catheter, acetic acid (1.30 liter) was added and heated to reflux. A mixed solution of methyl 4-acetyl-5-oxohexanoate (248.71 g, 1.34 mol) and diethyl aminomalonate hydrochloride (367.50 g, 1.74 mol) in acetic acid (0.85 L) was added dropwise over a period of 1 hour to the reaction mixture in reflux. After the dropwise addition, the reaction was continued in reflux for 2.5 hours. The reaction process was monitored by HPLC to confirm complete conversion. After the reaction mixture was cooled to room temperature, acetic acid was removed by distillation under reduced pressure. The resulting dark-colored crude product was mixed with water (4.5 liters), which was added slowly in batches and mechanically stirred for 1 hour. It was subsequently filtered and the filter cake was washed with water (1 liter). Recrystallization of the dark gray crude product was carried out by refluxing from a solvent mixture of ethanol/water (350 ml/350 ml) followed by cooling to 10 °C. The precipitate was separated by filtration and washed with ethanol/water (4 x 100 mL/100 mL). The product was dried under reduced pressure at room temperature to give compound (5) in light purple solid form (95.00 g, 28% yield).1H NMR (300 MHz, CDCl3): δ 1.34 (t, CH3), 2.21 (s, CH3), 2.27 (s, CH2), 2.43 (t, CH2), 2.70 (t, CH2), 3.66 (s, CH3), 4.29 (q, CH2), 8.60 (br s, NH2).
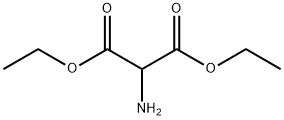
6829-40-9
97 suppliers
$45.00/5G

13984-53-7
41 suppliers
$60.00/50mg

2386-37-0
32 suppliers
$75.00/250mg
Yield:2386-37-0 80%
Reaction Conditions:
with acetic acidHeating;
References:
Paine, John B.;Dolphin, David [Journal of Organic Chemistry,1985,vol. 50,# 26,p. 5598 - 5604]

122676-34-0
0 suppliers
inquiry

2386-37-0
32 suppliers
$75.00/250mg

13984-53-7
41 suppliers
$60.00/50mg

16115-80-3
105 suppliers
$45.00/100mg

2386-37-0
32 suppliers
$75.00/250mg
2199-44-2
152 suppliers
$8.00/1g

2386-37-0
32 suppliers
$75.00/250mg
![1H-Pyrrole-2-carboxylic acid, 3,5-dimethyl-1-[(phenylmethoxy)methyl]-, ethyl ester](/CAS/20210305/GIF/122676-31-7.gif)
122676-31-7
0 suppliers
inquiry

2386-37-0
32 suppliers
$75.00/250mg