
tert-Butyl 4-(4-amino-2-fluorophenoxy)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(4-amino-2-fluorophenoxy)piperidine-1-carboxylate
- CAS Number:250372-00-0
- Molecular formula:C16H23FN2O3
- Molecular Weight:310.36

141-78-6
653 suppliers
$22.37/250ml

250371-88-1
19 suppliers
$294.00/250mg

250372-00-0
12 suppliers
$407.00/1g
Yield:250372-00-0 97%
Reaction Conditions:
palladium in methanol;hexane;
Steps:
R.23 4-(1-t-Butoxycarbonylpiperidin-4-yloxy)-3-fluoroaniline
Reference Example 23 4-(1-t-Butoxycarbonylpiperidin-4-yloxy)-3-fluoroaniline To a solution of 4-(1-t-butoxycarbonylpiperidin-4-yloxy)-3-fluoronitrobenzene (3.71 g) in methanol (50 ml) was added palladium on carbon (0.30 g) and the mixture was stirred under a hydrogen atmosphere at room temperature for 4 hours. The reaction mixture was filtered and the filtrate concentrated in vacuo. The residue was purified by chromatography on a silica gel column using hexane/ethyl acetate=1/1 as an eluant to give the desired compound (3.27 g, yield 97%) as a pale red solid. 1H NMR (500 MHz, CDCl3) δ ppm: 1.46 (9H, s), 1.72 (2H, m), 1.86 (2H, m), 3.23 (2H, m), 3.75 (2H, m), 4.17 (1H, m), 6.35 (1H, dd, J=8.5, 3.0), 6.44 (1H, dd, J=12.5, 3.0), 6.82 (1H, dd, J=9.0, 8.5).
References:
US6555556,2003,B1

250371-88-1
19 suppliers
$294.00/250mg

250372-00-0
12 suppliers
$407.00/1g
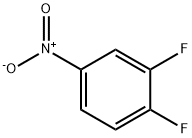
369-34-6
416 suppliers
$5.00/5g

250372-00-0
12 suppliers
$407.00/1g
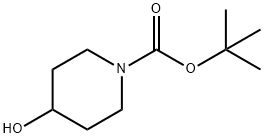
109384-19-2
471 suppliers
$5.00/1g

250372-00-0
12 suppliers
$407.00/1g
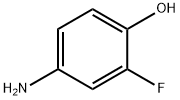
399-96-2
178 suppliers
$6.00/1g

109384-19-2
471 suppliers
$5.00/1g

250372-00-0
12 suppliers
$407.00/1g