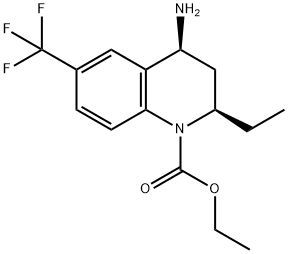
261947-64-2 synthesis
- Product Name:261947-64-2
- CAS Number:261947-64-2
- Molecular formula:C15H19F3N2O2
- Molecular Weight:316.32
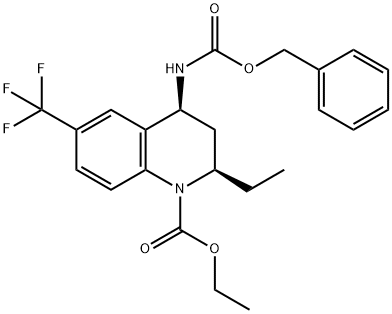
1140966-17-1
1 suppliers
inquiry

261947-64-2
9 suppliers
inquiry
Yield:261947-64-2 90%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;ammonium formate in methanol;water; for 2 h;Heating;
Steps:
4.1.6. Ethyl (2R,4S)-4-amino-2-ethyl-6-(trifluoromethyl)-3,4-dihydroquinoline-1(2H)-carboxylate (7)
To a solution of 6 (215 mg, 0.5 mmol) in methane (5 mL) wasadded ammonium format (80 mg, 1.25 mmol), the mixture wastreated with hydrogen over 10% Pd/C (20 mg, 50% water wet) at 40 °C for 2 h. The mixture was cooled to room temperature andfiltered through Celite, then the filtrate was concentrated in vacuoand the residue was purified by column chromatography (petroleumether: ethyl acetate 1:1) to provide 7 (113 mg, 90%) as a lightyellow oil. 1H NMR (300 MHz, CDCl3) d 7.74e7.71 (m, 1H), 7.57e7.44(m, 2H), 4.46e4.39 (m, 1H), 4.34e4.14 (m, 2H), 3.88e3.79 (m, 1H),2.59e2.45 (m, 1H), 1.71e1.62 (m, 1H), 1.53e1.39 (m, 1H), 1.33e1.24(m, 6H), 0.86 (t, J 7.4 Hz, 3H).
References:
Chang, Yongzhi;Zhou, Shuxi;Li, Enqin;Zhao, Wenfeng;Ji, Yanpeng;Wen, Xiaoan;Sun, Hongbin;Yuan, Haoliang [European Journal of Medicinal Chemistry,2017,vol. 126,p. 143 - 153]
84996-11-2
23 suppliers
inquiry

261947-64-2
9 suppliers
inquiry
![Pentanenitrile, 3-[[4-(trifluoromethyl)phenyl]amino]-, (3R)-](/CAS/20200331/GIF/474645-91-5.gif)
474645-91-5
1 suppliers
inquiry

261947-64-2
9 suppliers
inquiry
![3-[4-(TRIFLUOROMETHYL)ANILINO]PENTANAMIDE](/CAS/GIF/667937-05-5.gif)
667937-05-5
10 suppliers
$305.00/50mg

261947-64-2
9 suppliers
inquiry

474645-96-0
2 suppliers
inquiry

261947-64-2
9 suppliers
inquiry