
2-METHYL-3-(2-(METHYLTHIO)PYRIMIDIN-4-YL)IMIDAZO[1,2-A]PYRIDINE synthesis
- Product Name:2-METHYL-3-(2-(METHYLTHIO)PYRIMIDIN-4-YL)IMIDAZO[1,2-A]PYRIDINE
- CAS Number:264127-50-6
- Molecular formula:C13H12N4S
- Molecular Weight:256.33
![2-Propen-1-one, 3-(dimethylamino)-1-(2-methylimidazo[1,2-a]pyridin-3-yl)-](/CAS/20210305/GIF/328062-28-8.gif)
328062-28-8
0 suppliers
inquiry
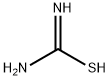
17356-08-0
2 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g
![2-METHYL-3-(2-(METHYLTHIO)PYRIMIDIN-4-YL)IMIDAZO[1,2-A]PYRIDINE](/CAS/20180529/GIF/264127-50-6.gif)
264127-50-6
6 suppliers
inquiry
Yield:264127-50-6 71%
Reaction Conditions:
Stage #1: 3-(3-dimethylaminoprop-2-en-1-oyl)-2-methylimidazo[1,2-a]pyridine;thioureawith sodium methylate in butan-1-ol at 85; for 2 h;
Stage #2: methyl iodide in butan-1-ol at 85; for 1 h;
Steps:
1 4-(2-Methylimidazo[1.2a]pyrid-3-yl)-2-methylthiopyrimidine
Method 1 4-(2-Methylimidazo[1.2a]pyrid-3-yl)-2-methylthiopyrimidine A mixture of 3-(3-dimethylaminoprop-2-en-1-oyl)-2-methylimidazo[1,2a]pyridine (Method 2) (20g, 87mmol), thiourea (6.52g, 86mmol) and sodium methoxide (1.19g, 22mmol) in butanol (220ml) was heated at 85°C for two hours under nitrogen. Methyl iodide (2ml, 32mmol) was added and the mixture heated at 85°C for a further 1 hour. Methanol was added and the volatiles were removed by evaporation. The residue was purified by chromatography eluding with ethyl acetate/methanol (100:0 increasing in polarity to 97:3) to give the title compound (16g, 71%). NMR: 2.59 (s, 1H), 2.62 (s, 3H), 7.10 (dd, 1H), 7.40 (dd, 1H), 7.42 (d, 1H), 7.63 (d, 1H), 8.62 (s, 1H), 9.54 (d, 1H), m/z: 257 [MH]+.
References:
EP1214318,2003,B1 Location in patent:Page/Page column 32
![3-ACETYL-2-METHYLIMIDAZO[1,2-A]PYRIDINE](/CAS/GIF/29096-60-4.gif)
29096-60-4
80 suppliers
$26.00/250mg
![2-METHYL-3-(2-(METHYLTHIO)PYRIMIDIN-4-YL)IMIDAZO[1,2-A]PYRIDINE](/CAS/20180529/GIF/264127-50-6.gif)
264127-50-6
6 suppliers
inquiry
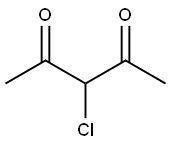
1694-29-7
116 suppliers
$40.00/10g
![2-METHYL-3-(2-(METHYLTHIO)PYRIMIDIN-4-YL)IMIDAZO[1,2-A]PYRIDINE](/CAS/20180529/GIF/264127-50-6.gif)
264127-50-6
6 suppliers
inquiry
![2(1H)-Pyrimidinethione, 4-(2-methylimidazo[1,2-a]pyridin-3-yl)-](/CAS/20210305/GIF/264606-78-2.gif)