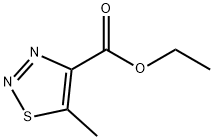
5-Methyl-1,2,3-thiadiazole-4-carboxylic acid ethyl ester synthesis
- Product Name:5-Methyl-1,2,3-thiadiazole-4-carboxylic acid ethyl ester
- CAS Number:29682-53-9
- Molecular formula:C6H8N2O2S
- Molecular Weight:172.2
2009-97-4
51 suppliers
$31.00/1g

29682-53-9
21 suppliers
$45.00/10mg
Yield:29682-53-9 84%
Reaction Conditions:
with Lawessons reagent in toluene at 100;Inert atmosphere;
Steps:
Step 2:
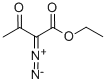
Lawesson’s reagent (127.45 g> 315-10 mmol) was added to a solution of ethyl 2- diazo-3-oxo-butanoate (41 g, 262.59 mmol) in toluene (600 mL). Then the mixture was stirred at ioo°C under N2for 8 hours. The mixture was poured into water (1 L) and extracted with ethyl acetate (2 L x 2). The combined organic layers were washed with water (1 L), dried over Na2SO4and filtered. The filtrate was concentrated to give the crude product which was purified by column chromatography on silica gel (petroleum ether : ethyl acetate = 100:0 to 80:20) to give ethyl 5-methylthiadiazole-4-carboxylate (40 g, 220.7 mmol, 84.0% yield, 95% purity) as a yellow solid.TH NMR (400 MHz, DMSO-d6) 4.55-8.48 (m, 2H), 2.92-2.89 (m, 3H), 1.50-1.45 (m, 3H).
References:
WO2022/167819,2022,A1 Location in patent:Page/Page column 204-205

141-97-9
823 suppliers
$5.00/25g

29682-53-9
21 suppliers
$45.00/10mg