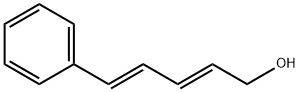
(2E,4E)-5-PHENYL-PENTA-2,4-DIEN-1-OL synthesis
- Product Name:(2E,4E)-5-PHENYL-PENTA-2,4-DIEN-1-OL
- CAS Number:58506-33-5
- Molecular formula:C11H12O
- Molecular Weight:160.21
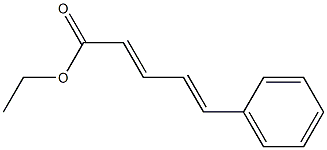
1552-95-0
0 suppliers
inquiry

58506-33-5
5 suppliers
inquiry
Yield: 69%
Reaction Conditions:
with diisobutylaluminium hydride in hexane;dichloromethane at -78; for 1.5 h;Inert atmosphere;
Steps:
General procedure A: Reduction of ester
General procedure: To a solution of ester in dry DCM at -78 °C under argon, DIBAL-H (1M solution in hexane) was added dropwise. The resulting mixture was stirred for 1.5 h at -78 °C and then quenched with 10% aqueous solution NaOH. The resulting mixture was warmed to room temperature and stirred for 1 h. The layers were separated and the aqueous layer was extracted with DCM (3 times). The combined organic phases were successively washed with brine, dried over magnesium sulfate, filtered and concentrated in vacuo. The crude alcohol was purified by flash column chromatography.
References:
Biletskyi, Bohdan;Tenaglia, Alphonse;Clavier, Hervé [Tetrahedron Letters,2018,vol. 59,# 2,p. 103 - 107] Location in patent:supporting information
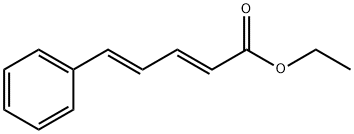
39806-16-1
4 suppliers
inquiry

58506-33-5
5 suppliers
inquiry
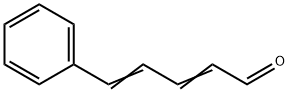
13466-40-5
15 suppliers
inquiry

58506-33-5
5 suppliers
inquiry
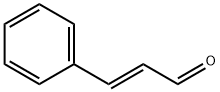
14371-10-9
334 suppliers
$6.00/25g

58506-33-5
5 suppliers
inquiry
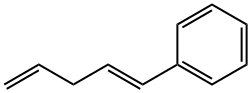
55666-17-6
0 suppliers
inquiry

58506-33-5
5 suppliers
inquiry