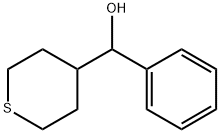
2H-Thiopyran-4-methanol, tetrahydro-α-phenyl- synthesis
- Product Name:2H-Thiopyran-4-methanol, tetrahydro-α-phenyl-
- CAS Number:1557244-40-2
- Molecular formula:C12H16OS
- Molecular Weight:208.32
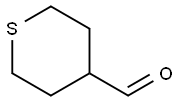
50675-19-9
73 suppliers
$45.00/50mg
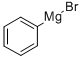
100-58-3
197 suppliers
$18.00/10ml

1557244-40-2
0 suppliers
inquiry
Yield:1557244-40-2 26%
Reaction Conditions:
in tetrahydrofuran at 0 - 20; for 12 h;Inert atmosphere;
Steps:
A55 Example A55: 4-((4-amino-lH-pyrazol-l-yl)(phenyl)methyl)tetrahydro-2H-thiopyran 1,1- dioxide
Example A55: 4-((4-amino-lH-pyrazol-l-yl)(phenyl)methyl)tetrahydro-2H-thiopyran 1,1- dioxide Into a 2-L 3 -necked round-bottom flask purged and maintained with a nitrogen atmosphere, was placed tetrahydro-2H-thiopyran-4-carbaldehyde (65 g, 499.20 mmol, 1.00 equiv) and tetrahydrofuran (300 mL). PhMgBr (1M, 750 mL, 1.50 equiv) was added dropwise to the stirred solution at 0 °C. The resulting solution was then stirred for 12 h at room temperature. The reaction progress was monitored by TLC with PE/DCM=2/1. The reaction was then quenched by the addition of 500 mL of saturated NH4C1 and extracted with 3x500 mL of ethyl acetate. The organic was washed with 3x500 mL of brine, dried over anhydrous sodium sulfate and concentrated under vacuum. The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1/20). This resulted in 27.0 g (26%) of phenyl(tetrahydro-2H-thiopyran- 4-yl)methanol as a yellow oil. The title compound was then prepared in an analogous manner to l-(3-(methylsulfonyl)- l-phenylpropyl)-lH-pyrazol-4-amine (Example A53), replacing 3-(methylsulfanyl)-l- phenylpropan- 1 -ol with phenyl(tetrahydro-2H-thiopyran-4-yl) .
References:
WO2014/23258,2014,A1 Location in patent:Page/Page column 78