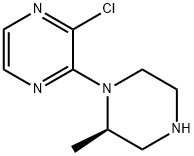
(2R)-1-(3-CHLORO-2-PYRAZINYL)-2-METHYLPIPERAZINE synthesis
- Product Name:(2R)-1-(3-CHLORO-2-PYRAZINYL)-2-METHYLPIPERAZINE
- CAS Number:313657-76-0
- Molecular formula:C9H13ClN4
- Molecular Weight:212.68
![Pyrazine, 2-chloro-3-[(2R)-2-methyl-4-(triphenylmethyl)-1-piperazinyl]-](/CAS/20210305/GIF/531503-76-1.gif)
531503-76-1
1 suppliers
inquiry

313657-76-0
15 suppliers
inquiry
Yield:313657-76-0 100%
Reaction Conditions:
with sulfuric acid in water;isopropyl alcohol at 77 - 83; for 0.5 h;
Steps:
8 EXAMPLE 8-Preparation of [ ERAZINE]
To the 1200 L reactor containing the product from Example 7 was added IPA (707 kg). Stirring at reflux [(77°C] to [83°C)] was continued until dissolution was complete. The solution in the 1200 L reactor was transferred to the 4000 L reactor with a IPA rinse (100 kg) and heated to reflux [(77°C] to [83°C).] A 10% sulfuric acid solution (giving a clean reaction) was prepared in the 4000 L receiver by adding water (245 L) and 98% H2SO4 (27 kg). The [H2SO4] solution was stirred for 10 minutes at [20°C] to [60°C] and transferred to the 4000 L reactor while maintaining the 4000 L reactor temperature between [60°C] to [85°C.] The [4000] L receiver was rinsed with water (25 L) sending the rinse to the 4000 L reactor. The 4000 L reactor was heated to reflux [(77°C] to [83°C).] The reaction was monitored by GC and was complete in 30 min. The contents in the 4000 L reactor were cooled to [35°C] to [40°C.] The IPA was removed under vacuum distillation. Water (1265 L) and [MTBE] (511 kg) was added to the 4000 L reactor. After stirring and settling, the aqueous layer was transferred into the 4000 L receiver. The [MTBE] layer in the 4000 L reactor was disposed. [MTBE] (511 kg) was added to the 4000 L receiver, the solution was stirred and settled for at least 15 min. The aqueous layer was transferred to the 4000 L reactor. The [MTBE] layer in the 4000 L receiver was disposed. The pH was adjusted to 9.5 to 10.5 using 47% [K2CO3] (206 kg) with a water rinse (50 L) of the add line. [CH2CI2] (2 x 765 L, [1] x 255 L) was used to extract the title compound from the 4000 L reactor sending the [CH2CI2] extractions into the 4000 L receiver. The organic solution in the 4000 L receiver was transferred to the 4000 L reactor with a [CH2C12] rinse (100 L). The CH2C12 was removed by distillation. Toluene (1000 L) was added to the 4000 L reactor and subsequently removed by vacuum distillation to afford a toluene solution of the title compound of 95.77% GC purity. The yield was quantitative. Summarizing the steps of Examples 5-8, the yield of the product from Example 8 was 67% starting from (R)-2-methylpiperazine, L-tartrate. According to D1, the yield of the same product was 60% starting from (R)-2-methylpiperazine, L-tartrate. The step described in [D 1] corresponding to the process in Example 8 was performed in a mixture of hot ethanol and 10% aqueous hydrochloric acid. 'H NMR (400 MHz, [CDC13)] : [6] 8.12 (1H, d, J = 2.6 Hz), 7.87 (1H, d, J = 2. 6 Hz), 4.18-4. 12 [(1H, M),] 3. 39 (2H, t, J = 3. 6 Hz), 3.12 [(1H,] dd, [J =] 12.2, 3. [6 HZ),] 3.06 [(1H,] dt, [J =] 12.2, 3.6 Hz), 3.02-2. 95 [(1H, M),] 2.81 [(1H,] dd, J = 12.2, 4.0 Hz), 1.90 [(1H,] br s), 1.21 (3H, d, [J = 6.] 6 Hz), 0.00 (TMS, reference). 13C NMR (100 MHz, CDCl3) : [6] 155.15 (s), 140.85 (s), 139.93 (d), 135.15 (d), 51.06 (d), [50.] 98 (t), 45.94 (t), 45.10 (t), 14.72 (q), 0.00 (TMS, reference). IR (liq. ) 2940,2389 (w), 2149 (w), 1996 (w), 1556 (s), 1504 (s), 1459 (s), 1440 (s), 1415 (s), 1367,1330 (s), 1132 (s), 1107 (s), 1100 (s), 1049 (s), cm-l. [[A]] [25D =-43°] (c 0.59, [CH2C12).] HRMS (FAB) calcd for [COHI3CLN4] [+HI] 213.0907, found 213.0909. Anal. Calcd for [C9HL3CIN4] : C, 50.83 ; H, 6.16 ; N, 26.34. Found: C, 50.48 ; H, 6.19 ; N, 26.47.
References:
WO2004/829,2003,A1 Location in patent:Page 28-29
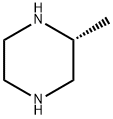
75336-86-6
242 suppliers
$9.00/5g

313657-76-0
15 suppliers
inquiry
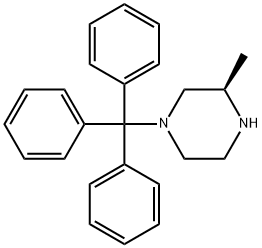
313657-75-9
46 suppliers
$51.20/250mg

313657-76-0
15 suppliers
inquiry
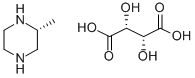
126458-16-0
17 suppliers
inquiry

313657-76-0
15 suppliers
inquiry
109-07-9
382 suppliers
$5.00/5g

313657-76-0
15 suppliers
inquiry