
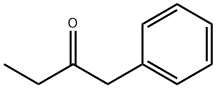
(2R)-1-phenylbutan-2-ol synthesis
- Product Name:(2R)-1-phenylbutan-2-ol
- CAS Number:29393-19-9
- Molecular formula:C10H14O
- Molecular Weight:150.22
1007-32-5
132 suppliers
$55.00/1g

29393-19-9
5 suppliers
inquiry
Yield:29393-19-9 79.8%
Reaction Conditions:
with Rhizopus arrhizus in ethanol;water at 24 - 25; for 72 h;Microbiological reaction;Enzymatic reaction;enantioselective reaction;
Steps:
(R)-1-Phenyl-2-butanol 5a
General procedure: The laboratory scale-up bioreduction of ketones 1-7 was carried out as follows. After 72h of fermentation, R. arrhizus mycelia were separated from the culture broth. The 10% wet mycelia were put in a 1.5L sterilized fresh culture medium as working volume in 5L Erlenmeyer flask under aseptic conditions and incubated at room temperature for 72h under static conditions. After the growth of the fungus, the substrate (1g) in ethanol was added directly to the medium and then incubated at room temperature on a rotary shaker (100rpm) for 8days. At the end of the incubation period, the mycelia were separated by filtration. Mycelia was washed with water and then the combined aqueous medium was extracted with chloroform. The chloroform extract washed with water and dried over Na2SO4. After removal of the solvent under reduced pressure, the product alcohol was isolated, purified, and characterized as described earlier. The absolute configuration was determined by the sign of the specific rotation and comparison with the literature data. Isolated yield: 0.81g, [α]D24=-28.7 (c 0.436, CHCl3) 97.1% ee {lit.2i=-31.1 (c 2.82, CHCl3), 94.9% ee}; 1H NMR (CDCl3, 200MHz): δ 1.05 (t, 3H, CH3), 1.52-1.77 (m, 2H, CH2), 1.80 (s, 1H, OH), 2.93 (m, 2H, CH2), 3.76-3.83 (m, 1H, CHOH), 7.24-7.42 (m, 5H, Ar); 13C NMR (CDCl3, 200MHz, ppm): 9.91, 29.34, 43.41, 73.82, 126.16, 128.30, 129.29, 138.63; methoxyl resonances of MTPA ester, 1H NMR: δ 3.46 [minor, (S)-isomer] and 3.43 [major, (R)-isomer]
References:
Salvi, Neeta A.;Chattopadhyay, Subrata [Tetrahedron Asymmetry,2016,vol. 27,# 4-5,p. 188 - 192]
![Benzene, 1-chloro-4-[(R)-[(1R)-1-chloro-2-phenylethyl]sulfinyl]-](/CAS/20210305/GIF/222016-68-4.gif)
222016-68-4
0 suppliers
inquiry

29393-19-9
5 suppliers
inquiry