
(2S)-2-AMINO-4,4-DIMETHYLPENTAN-1-OL synthesis
- Product Name:(2S)-2-AMINO-4,4-DIMETHYLPENTAN-1-OL
- CAS Number:154092-62-3
- Molecular formula:C7H17NO
- Molecular Weight:131.22
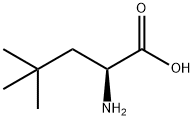
57224-50-7
108 suppliers
$48.00/250mg

154092-62-3
5 suppliers
inquiry
Yield:154092-62-3 84%
Reaction Conditions:
with lithium borohydride;chloro-trimethyl-silane in tetrahydrofuran at 0 - 20; for 24 h;Inert atmosphere;
Steps:
1
Step 1: (S)-2-Amino-4,4-dimethyl-pentan-1-ol To a stirred suspension of lithium borohydride (0.75 g, 34.4 mmol, 2.0 equiv.) in tetrahydrofuran (60 mL) was added trimethylsilyl chloride (7.48 g, 68.85 mmol, 4.0 equiv.) slowly over a period of 5 minutes at 0° C. under argon. To the reaction mixture was added (S)-2-amino-4,4-dimethyl-pentanoic acid (available from Chem-Impex International, Inc., Wood Dale, Ill., USA; 2.50 g, 17.2 mmol, 1.0 equiv.) in portions over a span of 10 minutes at the same temperature and the reaction was allowed to stir for 24 hours at room temperature. The reaction mixture was quenched with the slow addition of methanol (50 mL) at 0° C., and volatiles were removed by vacuum distillation. The obtained residue was treated with an aqueous solution of 20% potassium hydroxide (w/v; 10 mL) and extracted with dichloromethane (3*20 mL). The combined organic phases were dried over anhydrous sodium sulfate and concentrated under reduced pressure to give (S)-2-amino-4,4-dimethyl-pentan-1-ol (1.9 g, 84%).
References:
US2011/124686,2011,A1 Location in patent:Page/Page column 24