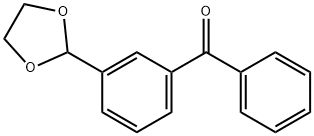
3-(1,3-DIOXOLAN-2-YL)BENZOPHENONE synthesis
- Product Name:3-(1,3-DIOXOLAN-2-YL)BENZOPHENONE
- CAS Number:85366-46-7
- Molecular formula:C16H14O3
- Molecular Weight:254.28
Yield:85366-46-7 367 mg
Reaction Conditions:
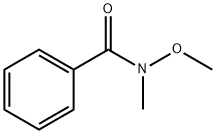
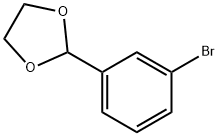
Stage #1: 3-(1,3-dioxolan-2-yl)-phenylbromidewith n-butyllithium in tetrahydrofuran at -70; for 1 h;
Stage #2: N-methoxy-N-methylbenzamide in tetrahydrofuran at -70; for 2 h;
Steps:
3 Example 3
The preparation method is as follows:Ethylene glycol acetal (458 mg, 2 mmol) of 3-bromobenzaldehyde,Dissolved in 5 mL of dry tetrahydrofuran,N-Butyllithium (0.8 mL, 2 mmol) was slowly added dropwise at -70 ° C,After 1 hour, N-methyl-N-methoxybenzamide (280 mg, 1.7 mmol) was added slowly at -70 ° C,The reaction was continued at -70 ° C for 2 hours,Add 5mL saturated brine to quench the reaction,The organic phase was extracted with ethyl acetate (10 mL * 3)The combined organic phases are dried over anhydrous sodium sulphate.Spin the solvent,Column chromatography (petroleum ether: ethyl acetate = 20: 1 as eluant),The reaction product 367mg.The reaction product was dissolved in 5 mL of dry methanol,1mL 6N hydrochloric acid was added at room temperature, the reaction 1 hour, the reaction was quenched by adding saturated sodium bicarbonate solution,The organic phase was extracted with ethyl acetate (10 mL * 3). The organic phases were combined and dried over anhydrous sodium sulfate.The solvent was spin-dried and separated by column chromatography (petroleum ether: ethyl acetate = 20: 1 as eluant)The reaction product3-benzoylbenzaldehyde 266 mg, 75% over two steps.
References:
CN107286137,2017,A Location in patent:Paragraph 0097; 0098; 0099; 0100
17789-14-9
146 suppliers
$6.00/1g

100-52-7
977 suppliers
$20.37/250g

85366-46-7
12 suppliers
inquiry

17789-14-9
146 suppliers
$6.00/1g

85366-46-7
12 suppliers
inquiry

100-52-7
977 suppliers
$20.37/250g

85366-46-7
12 suppliers
inquiry