
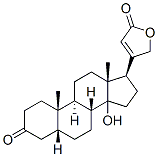
3α,14β-Dihydroxy-5β,14β-carda-20(22)-enolide synthesis
- Product Name:3α,14β-Dihydroxy-5β,14β-carda-20(22)-enolide
- CAS Number:545-52-8
- Molecular formula:C23H34O4
- Molecular Weight:374.51
1102-88-1
1 suppliers
inquiry

545-52-8
4 suppliers
inquiry
Yield:545-52-8 94%
Reaction Conditions:
with sodium tetrahydroborate in 1,4-dioxane;water at 5; for 0.5 h;
Steps:
4.2.3. Reduction of digitoxigenone (2) to 3α-digitoxigenin (3)
1 g of digitoxigenone (2) was dissolved in 40 mL dioxane and 10 mL water. Then, 365 mg NaBH4 in 37.5 mL of 80% dioxane (30 mL dioxane 7.5 mL H2O) were added to the solution and stirred for 30 min at 5 °C. The reaction was neutralized with 30 mL of 5% acetic acid and the pH was adjusted to 5. Hence, the reaction color turned from yellow to white after adding the acid. For stopping the reaction and avoiding the formation of additional reaction products, the fast reducing of dioxane under vacuo is essential. The aqueous residue was extracted with 5 Χ 30 mL chloroform/ethanol(3:1) and the organic layer was washed 3 30 mL of water, and the organic layer was dried over anhydrous sodium sulfate and evaporated to dryness. 4.2.3.1. 3α-digitoxigenin (3). Yield: 94%; mp 266.4-269.5 °C (Lit:268-270 C [76]). [α]D30 5.7 (c 0.35; MeOH). Lit:[a]D22 26.8 (c 0.33; MeOH [77]). IR: ü 3419-3507 cm-1 (OH), 2859-2929 cm1(C-H sp3), 1733 cm-1 (C=O), 1632 cm-1 (C=C), 1036 cm1 (CeO).1H NMR (400 MHz, DMSO-d6): δ 0.77 (s, 3H, CH3, H-18), 0.85 (s, 3H,CH3, H-19), 0.89-1.83 (m, 19H, H-1, H-2, H-4, H-5, H-6, H-7, H-8, H-9, H-11, H-12, H-16), 2.00e2.08 (m, 2H, H-15), 2.72e2.74 (m, 1H, H-17), 3.36e3.41 (m, 1H, H-3), 4.06 (s, 1H, OH), 4.43 (d, J 4.4 Hz, 1H,OH), 4.87 (d, J 18.6 Hz, 1H, H-21a), 4.97 (d, J 18.2 Hz, 1H, H-21b),5.90 (s, 1H, H-22). 13C NMR (100 MHz, DMSO-d6): 15.7 (CH3, C-18),20.6 (CH2, C-7), 21.2 (CH2, C-11), 23.1 (CH3, C-19), 26.4 (CH2, C-6),26.9 (CH2, C-16), 30.4 (CH2, C-2), 32.2 (CH2, C-1), 34.5 (C0, C-10),34.9 (CH2, C-15), 35.5 (CH, C-9), 36.2 (CH2, C-4), 39.0 (CH2, C-12),41.0 (CH, C-5), 41.3 (CH, C-8), 49.4 (C0, C-13), 50.2 (CH, C-17), 69.8(CH, C-3), 73.1 (CH2, C-21), 83.7 (C0, C-14), 116.2 (CH, C-22), 173.8(C]O, C-23), 176.3 (C0, C-20); HRMS-ESI: calcd for C23H34O4[MH] 375.5211, found: 375.45.
References:
Boff, Laurita;Munkert, Jennifer;Ottoni, Flaviano Melo;Zanchett Schneider, Naira Fernanda;Ramos, Gabriela Silva;Kreis, Wolfgang;Fernandes de Andrade, Saulo;Dias de Souza Filho, José;Braga, Fern?o Castro;Alves, Ricardo José;Maia de Pádua, Rodrigo;Oliveira Sim?es, Cláudia Maria [European Journal of Medicinal Chemistry,2019,vol. 167,p. 546 - 561]
143-62-4
126 suppliers
$39.00/5mg

545-52-8
4 suppliers
inquiry
71-63-6
238 suppliers
$39.00/100mg

545-52-8
4 suppliers
inquiry