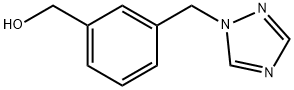
[3-(1H-1,2,4-TRIAZOL-1-YLMETHYL)PHENYL]METHANOL synthesis
- Product Name:[3-(1H-1,2,4-TRIAZOL-1-YLMETHYL)PHENYL]METHANOL
- CAS Number:871825-54-6
- Molecular formula:C10H11N3O
- Molecular Weight:189.21
857284-24-3
11 suppliers
inquiry
![[3-(1H-1,2,4-TRIAZOL-1-YLMETHYL)PHENYL]METHANOL](/CAS/GIF/871825-54-6.gif)
871825-54-6
18 suppliers
$45.00/10mg
Yield:871825-54-6 22%
Reaction Conditions:
with sodium tetrahydroborate at 80; for 16 h;Solvent: polyethylene glycol;
Steps:
(3-((lH-l,2,4-TriazoI-l-yI)methyl)phenyI)methanol (TJA02011) C10H11N3O MW 189.21. A 25 mL r.b. flask was loaded with TJA02008 (0.500 g, 2.36 mmol) and polyethylene glycol 400 (6.0 g). The mixture was heated to 80 0C with stirring until a solution had formed. Sodium borahydride (0.261 g, 6.91 mmol) was added carefully resulting in evolution of gas. The reaction mixture was stirred vigorously at 80 0C for 16 h. Extremely viscous glue formed that gradually dissolved in dichloromethane (50 mL) with heating (40 0C). This solution was washed with IM HCl(aq) (10 mL) and then carefully neutralised with sodium bicarbonate. Washed with distilled water (50 mL x 4) and brine (50 mL), separated and dried over MgSO4. Solvent removed in vacuo to leave a viscous yellow oil. Flash chromatography (20 g column, meihodβ) eluted the title compound as a colourless viscous oil (0.101 g, 22 %), R/. 0.24 (ethyl acetate); 1H NMR (270 MHz, CDCl3) δ 2.53 (IH5 bs, ArCH2OH)5 4.66 (2η, s, ArCH2OH), 5.30 (2H3 s, ArCH2N), 7.14-7.17 (IH5 m, ArH)5 7.29-7.37 (2H, m, ArH)5 7.89 (IH, s, C2H2N3) and 8.00 (IH5 s, C2H2N3);
References:
WO2007/68905,2007,A1 Location in patent:Page/Page column 134-135