
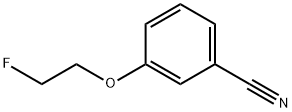
3-(2-Fluoro-ethoxy)-benzylamine synthesis
- Product Name:3-(2-Fluoro-ethoxy)-benzylamine
- CAS Number:1037078-87-7
- Molecular formula:C9H12FNO
- Molecular Weight:169.2
132838-24-5
2 suppliers
inquiry

1037078-87-7
5 suppliers
inquiry
Yield:1037078-87-7 100%
Reaction Conditions:
Stage #1: 3-(2-fluoroethoxy)benzonitrilewith lithium aluminium tetrahydride in tetrahydrofuran at 0; for 1.5 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran; for 0.333333 h;
Steps:
9.B
Lithium aluminum hydride (0.67 g, 17.9 mmol) was charged to a flame dried 50 mL round bottom flask and the flask was cooled to 0 0C. Tetrahydrofuran (14 mL) was added to the flask, followed by the product of Part A (1.18g, 7.14 mmol). The ice bath was removed and the mixture stirred for 1.5 h, cooled to 0 0C, and quenched by adding water (0.68 mL) and 15% NaOH (0.68 mL), followed by an addition of water (2.04 mL). This mixture was stirred for 20 min, filtered, and the filtrate was concentrated to afford 1.22 g (100%) of the product as an oil. This oil was pure by NMR. 1H NMR (300 MHz, CDCl3): δ 7.25 (m, IH), 6.9 (m, 2H), 6.8 (m, IH), 4.75 (t of d, 2H, J = 4.2, 47 Hz), 4.25 (t of d, 2H, J = 4.2, 28 Hz), 3.8 (s, 2H). 13C NMR (75 MHz, CDCl3): δ 158.6, 145.1, 129.5, 119.9, 113.3, 112.8, 81.9 (d, J = 169 Hz), 67 (d, J = 21 Hz), 46.3.
References:
WO2008/83056,2008,A2 Location in patent:Page/Page column 37