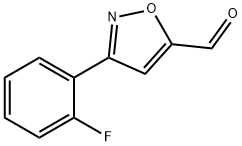
3-(2-FLUORO-PHENYL)-ISOXAZOLE-5-CARBALDEHYDE synthesis
- Product Name:3-(2-FLUORO-PHENYL)-ISOXAZOLE-5-CARBALDEHYDE
- CAS Number:808740-52-5
- Molecular formula:C10H6FNO2
- Molecular Weight:191.16
![[3-(2-FLUORO-PHENYL)-ISOXAZOL-5-YL]-METHANOL](/CAS/GIF/953046-62-3.gif)
953046-62-3
50 suppliers
$45.00/10mg

808740-52-5
37 suppliers
$45.00/50mg
Yield:808740-52-5 88%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;iodine;sodium hydrogencarbonate in ethanol;water;benzene at 20; for 9 h;
Steps:
4.2. General synthesis process for 3-substituted isoxazole-5-carbaldehydes
General procedure: 3-Substituted isoxazole-5-carbaldehydes were synthesized according to reported procedures.37 (3-Phhenylisoxazol-5-yl)methanols (5.0 mmol) were dissolved into benzene (10 mL). An aqueous solution of sodium bicarbonate (13 mL, 1.2 mol/L) was added into the benzene slurry at room temperature. Then, the mixed solid TEMPO (0.5 mmol) was added and solid iodine (10 mmol) dissolved in alcohol was added into the reaction mixture. The reaction mixture was then aged for 10-12 h at room temperature; the reaction was monitored by TLC. The crude product was diluted with ethyl acetate (15 mL). The batch was washed with Na2S2O3 and transferred to a separatory funnel, and the aqueous layer was extracted with ethyl acetate (210 mL). The organic layers were mixed and dried over anhydrous sodium sulfate for 30 min, filtrated and evaporated under vacuum to give the crude product which was purified by column chromatography (silica gel, 200e300 mesh) using petroleum ether/ethyl acetate(4r5: 1) to furnish the product. The yields of obtained 3-substituted isoxazole-5-carbaldehydes products were 58-92%.
References:
Zhang, Dawei;Zhang, Yumin;Zhao, Tianqi;Li, Jing;Hou, Yaya;Gu, Qiang [Tetrahedron,2016,vol. 72,# 22,p. 2979 - 2987]
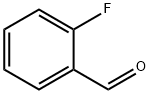
446-52-6
440 suppliers
$6.00/25g

808740-52-5
37 suppliers
$45.00/50mg