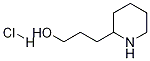
3-(2-Piperidyl)-1-propanol Hydrochloride synthesis
- Product Name:3-(2-Piperidyl)-1-propanol Hydrochloride
- CAS Number:90226-88-3
- Molecular formula:C8H18ClNO
- Molecular Weight:179.6876
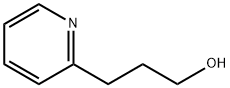
2859-68-9
165 suppliers
$14.14/250mgs:
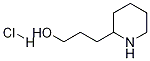
90226-88-3
27 suppliers
$65.00/50mg
Yield:90226-88-3 94.8%
Reaction Conditions:
with hydrogenchloride;platinum(IV) oxide;hydrogen in ethanol at 20; under 2327.23 Torr; for 16 h;
Steps:
7.A A: 3-(Piperidin-2-yl)propan-l-ol hydrochloride (compound 21)
A: 3-(Piperidin-2-yl)propan-l-ol hydrochloride (compound 21) To a stirred solution of compound 20 (5 g, 36.4 mmol) in ethanol (32 mL) was added concentrated HCl (3.2 mL) and the reaction mixture was degassed with N2 for 15 minutes. Platinum oxide (Pt02; 1 g) was then added and degassed for 5 minutes. Finally, the reaction mixture was hydrogenated at rt in a Parr apparatus for 16 hours under 45 psi H2 pressure. The reaction mixture was filtered through the Celite reagent, and was washed with ethanol. The filtrate was concentrated to yield the crude product 21 which was used as such for the next step. Yield: 6.2 g (94.8%); 1H-NMR (400 MHz, DMSO-<): δ 8.88 (brs, 1 H), 8.71 (brs, 1 H), 4.57 (s, 1 H), 3.40 (d, J= 4 Hz, 2 H), 3.17 (d, J= 12 Hz, 1 H), 2.96 (brs, 1 H), 2.81-2.79 (m, 1 H), 1.84 (d, J= 13 Hz, 1 H), 1.71-1.65 (m, 3 H), 1.62-1.58 (m, 1 H), 1.56-1.43 (m, 3 H), 1.40-1.38 (m, 1 H)
References:
WO2014/28675,2014,A1 Location in patent:Page/Page column 113