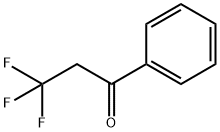
3,3,3-trifluoro-1-phenylpropan-1-ol synthesis
- Product Name:3,3,3-trifluoro-1-phenylpropan-1-ol
- CAS Number:2340-22-9
- Molecular formula:C9H9F3O
- Molecular Weight:190.16
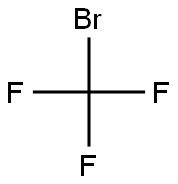
709-21-7
39 suppliers
$45.00/250mg

2340-22-9
9 suppliers
inquiry
Yield:2340-22-9 100%
Reaction Conditions:
with C17H28ClIrN2OS;hydrogen;sodium methylate in methanol at 40 - 42; under 18751.9 Torr; for 3 h;Reagent/catalyst;
Steps:
10 Example 10. Catalytic Hydrogenation of Aromatic Ketones (Scheme 16).
The results are summarized in Table 3. Again, the results are focusing on two iridium complexes to verify the effect of methylation of the NH ligand. (0609) (0610) Scheme 16. (0611) Table 3. Catalytic Hydrogenation of Aromatic Ketones.' (0612) Run cat subs S/C MeONa, Solv time, h temp, conv. yield, mol % °C %b %b lc N-4 Kl 5 000 5 MeOH 0.5d 40-41 100 100 (0613) 2C N-5 Kl 5 000 5 MeOH 0.5d 40 100 100 (0614) 3C N-4 Kl 50 000 5 MeOH 3 40-41 58 58 (0615) 4C N-5 Kl 50 000 5 MeOH 3 40 >99 >99 (0616) 5 N-4 K2 50 000 5 MeOH 3 40-42 9 9 (0617) 6 N-5 K2 50 000 5 MeOH 3 40 26 26 (0618) 7 N-4 K3 1 000 5 MeOH 1 40 >99 >99 (0619) 8 N-5 K3 1 000 5 MeOH 1 40 >99 >99 (0620) 9 N-4 K3 50 000 5 MeOH 3 40-42 25 25 (0621) 10 N-5 K3 50 000 5 MeOH 3 40-42 27 27 (0622) 11 N-4 K4 50 000 5 MeOH 3 40-43 ~0 ~0 (0623) 12 N-5 K4 50 000 5 MeOH 3 40-43 ~0 ~0 (0624) 13 N-4 K5 1 000 5 MeOH 3 40 ~0 ~0 (0625) 14 N-5 K5 1 000 5 MeOH 3 40 ~0 ~0 (0626) 15 N-4 K6 1 000 5 MeOH 3 40-42 100 100 (0627) 16 N-5 K6 1 000 5 MeOH 3 40-42 100 100 (0628) Standard reaction conditions: substrate (10 mmol), solvent (5 mL), 50 mL Parr autoclave. *NMR (1H or 19F, rd = 10 s). cEach run performed at least twice, see SI for details. ^Essentially completed after 20 min based on drop in pressure.
References:
WO2015/191505,2015,A1 Location in patent:Page/Page column 115-116
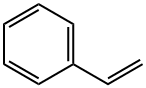
100-42-5
669 suppliers
$20.00/25mL
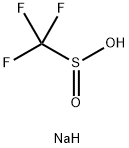
2926-29-6
311 suppliers
$6.00/5g

709-21-7
39 suppliers
$45.00/250mg

2340-22-9
9 suppliers
inquiry