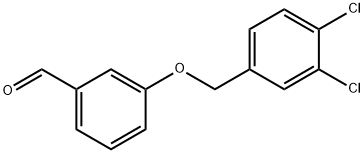
3-[(3,4-DICHLOROBENZYL)OXY]BENZALDEHYDE synthesis
- Product Name:3-[(3,4-DICHLOROBENZYL)OXY]BENZALDEHYDE
- CAS Number:588715-60-0
- Molecular formula:C14H10Cl2O2
- Molecular Weight:281.13

100-83-4
647 suppliers
$5.00/10g
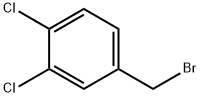
18880-04-1
158 suppliers
$10.00/5g
![3-[(3,4-DICHLOROBENZYL)OXY]BENZALDEHYDE](/CAS/GIF/588715-60-0.gif)
588715-60-0
16 suppliers
$75.00/500mg
Yield:588715-60-0 100%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 3 h;Inert atmosphere;
Steps:
SI-1.1. 3-{3-[(3,4-Dichlorobenzyl)oxy]phenyl}-3-ethoxypropanoicacid (23)
To a solution of 3-hydroxybenzaldehyde (1.53 g, 12.5 mmol)in DMF (30 mL), 3,4-dichlorobenzyl bromide (3.00 g, 12.5 mmol) and K2CO3(3.45 g, 25.0 mmol) were added at room temperature, and stirred under N2atmosphere for 3 h. Water was added to the reaction mixture, and followed byextraction with AcOEt. The organic layer was washed with brine, and dried over Na2SO4and filtered. After the solvent was distilled off under reduced pressure, 3-[(3,4-dichlorobenzyl)oxy]benzaldehydewas obtained as a white solid (3.52 g, 100%).To a solution of AcOEt (1.65 g, 2.34 mol) in THF (30 mL), alithium bis(trimethylsilyl)amide THF solution (1.23 M, 19.0 mL, 2.34 mol) wasadded at -78 oC. The reaction mixture was stirred under N2atmosphere at -78 oC for 1 h. To the reaction mixture, 3-[(3,4-dichlorobenzyl)oxy]benzaldehyde(3.52 g, 12.5 mmol) in THF (10 mL) was added, and stirred t -78 oC for1 h. To the reaction solution, saturated NH4Cl aq. was added at -78 oC.The organic material was extracted with diehtyl ether. The organic layer waswashed with water and brine, and dried over Na2SO4 andfiltered. After the solvent was distilled off under reduced pressure, theresidue was purified by silica gel column chromatography (hexane:AcOEt = 100:0to 80:20 (v/v)), ethyl3-{3-[(3,4-dichlorobenzyl)oxy]phenyl}-3-hydroxypropanoate was obtained ascolorless oil (4.01 g, yield 87 %).To a solution of ethyl3-{3-[(3,4-dichlorobenzyl)oxy]phenyl}-3-hydroxypropanoate (4.01 g, 10.7 mmol)in toluene (100 mL), EtI (2.60 mL, 32.6 mmol) and Ag2O (7.55 g, 32.6mmol) were added. The reaction mixture was stirred for 2 h at 100 oCunder N2 atmosphere. After cooling to room temperature, the reactionsolution was filtered, and the solvent was distilled off under reduced pressure.The residue was purified by silica gel column chromatography (hexane/AcOEt =100:0 to 20:80 (v/v)), ethyl3-{3-[(3,4-dichlorobenzyl)oxy]phenyl}-3-ethoxypropanoate was obtained ascolorless oil (2.76 g, yield 64%).To a solution of 3-{3-[(3,4-dichlorobenzyl)oxy]phenyl}-3-ethoxypropanoate(260 mg, 0.654 mmol) in THF (3.0 mL) and EtOH (3.0 mL), 2 N NaOH aq. (1.0 mL)was added at room temperature, and stirred for 6 h. The solvent was distilledoff under reduced pressure. 2 N HCl aq. (3.0 mL) was added to the reactionmixture, and followed by extraction with AcOEt. The organic layer was washedwith brine and dried over Na2SO4 and filtered. Thesolvent was distilled off under reduced pressure. The residue was purified bysilica gel column chromatography (hexane/AcOEt=100:0 to 0:100 (v/v)), the titlecompound was obtained as colorless oil (240 mg, 100%).
References:
Takano, Rieko;Yoshida, Masao;Inoue, Masahiro;Honda, Takeshi;Nakashima, Ryutaro;Matsumoto, Koji;Yano, Tatsuya;Ogata, Tsuneaki;Watanabe, Nobuaki;Hirouchi, Masakazu;Kimura, Takako;Toda, Narihiro [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 17,p. 5546 - 5565] Location in patent:supporting information