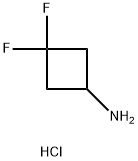
3,3-DIFLUOROCYCLOBUTANAMINE HYDROCHLORIDE synthesis
- Product Name:3,3-DIFLUOROCYCLOBUTANAMINE HYDROCHLORIDE
- CAS Number:637031-93-7
- Molecular formula:C4H8ClF2N
- Molecular Weight:143.56
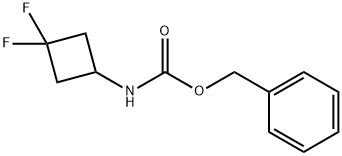
939399-53-8

637031-93-7
Step C: Synthesis of 3,3-difluorocyclobutylamine hydrochloride. A mixture of benzyl (3,3-difluorocyclobutyl)carbamate (1.47 g, 6.1 mmol) and 10% Pd/C (1 g) in methanol (20 mL) was subjected to a hydrogen atmosphere (1 atm) and stirred overnight at room temperature. After completion of the reaction, the mixture was filtered through a diatomaceous earth pad and washed with methanol. Concentrated hydrochloric acid (2 mL) was added to the filtrate, followed by evaporation of the solvent under vacuum to afford the target product 3,3-difluorocyclobutanamine hydrochloride (0.8 g, 85% yield). The product was characterized by 1H NMR (400 MHz, DMSO-d6): δ 8.60 (m, 3H), 3.64 (m, 1H), 2.89 (m, 4H).
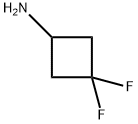
791061-00-2
79 suppliers
$8.00/250mg

637031-93-7
241 suppliers
$6.00/100mg
Yield:637031-93-7 95%
Reaction Conditions:
with hydrogenchloride in ethanol;water at 10 - 15; for 8 h;Solvent;
Steps:
7; 8 Example 7: Synthesis of Compound 6
Add compound 5 (90g, 0.84mol) synthesized in Example 4 to a 250mL reaction flask, add 4M hydrochloric acid ethanol solution (540g) at 10-15°C, stir for 8 hours, concentrate under reduced pressure to dryness, add ethyl acetate ( 450 mL) was stirred for 1 hour, then filtered and dried in vacuum at 50°C to obtain 114.3 g of a white solid with a yield of 95% and a purity of 100%.
References:
CN112279785, 2021, A Location in patent:Paragraph 0034; 0062-0065

939399-53-8
20 suppliers
inquiry

637031-93-7
241 suppliers
$6.00/100mg
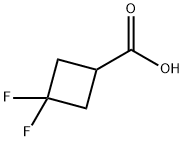
107496-54-8
217 suppliers
$29.00/1g

637031-93-7
241 suppliers
$6.00/100mg
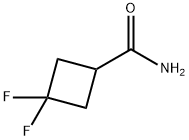
86770-82-3
29 suppliers
inquiry

637031-93-7
241 suppliers
$6.00/100mg
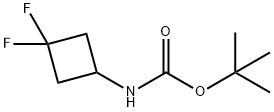
1029720-19-1
51 suppliers
$219.00/5g

637031-93-7
241 suppliers
$6.00/100mg