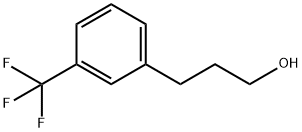
3-(3-(Trifluoromethyl)phenyl)propan-1-ol synthesis
- Product Name:3-(3-(Trifluoromethyl)phenyl)propan-1-ol
- CAS Number:78573-45-2
- Molecular formula:C10H11F3O
- Molecular Weight:204.19
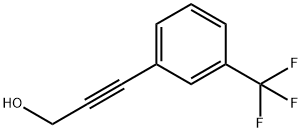
65126-85-4

78573-45-2
To a solution of 14.5 g (72.5 mmol) of purified 3-(3-trifluoromethylphenyl)propargyl alcohol in 50 mL of 2-propanol was added 0.38 g of 10% Pd/C (Selcat Q6). The reaction mixture was subjected to hydrogenation at a temperature of 42-45°C and a pressure of 5 bar until all feedstocks were completely converted (about 5 hours). Upon completion of the reaction, it was filtered to remove the catalyst and the catalyst was washed with a small amount of 2-propanol. Subsequently, the filtrate was concentrated by evaporation under reduced pressure. The crude product obtained (14.5 g, 100.0%) was purified by distillation under reduced pressure, resulting in 10.5 g (yield: 72.1%) of the target product 3-(3-trifluoromethylphenyl)propanol as an almost colorless oil (boiling point: 58-60 °C/1.1-1.5 mbar).
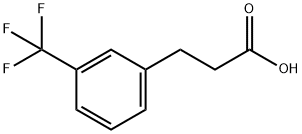
585-50-2
324 suppliers
$19.00/1g

78573-45-2
312 suppliers
$10.00/1g
Yield:78573-45-2 94%
Reaction Conditions:
Stage #1:3-(3-trifluoromethylphenyl)propanoic acid with borane-THF in tetrahydrofuran at 0 - 20; for 25 h;
Stage #2: with water in tetrahydrofuran;methanol
Steps:
3-(3-Trifluoromethyl-phenyl)-propionic acid (12.4 g, 57 mmol) was dissolved in THF (160 ml) in an inert atmosphere and cooled to 0° C. followed by slow addition of borane in THF (85 ml, 85 mmol). Stirring was continued at 0° C. for 1 h and at rt for 24 h. The mixture was cooled to 0° C. and methanol (100 ml) and water (100 ml) was carefully added. The organic solvents were removed under reduced pressure. The remaining aqueous layer was extracted with DCM (3*100 ml). The combined organic layers were washed with brine, dried over magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by flash chromatography (silicagel, DCM/methanol=9/1) to give 10.97 g (94%) of 3-(3-trifluoromethyl-phenyl)-propan-1-ol. LC-MS: tR=0.87 min; [M+H]+=no ionisation.
References:
Actelion Pharmaceuticals Ltd. US2009/99228, 2009, A1 Location in patent:Page/Page column 11; 18

65126-85-4
20 suppliers
$110.00/50mg

78573-45-2
312 suppliers
$10.00/1g
64189-17-9
9 suppliers
inquiry

78573-45-2
312 suppliers
$10.00/1g
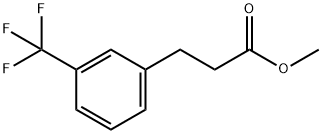
294856-02-3
27 suppliers
inquiry

78573-45-2
312 suppliers
$10.00/1g
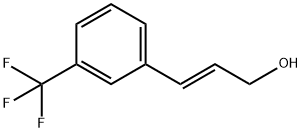
113048-69-4
20 suppliers
inquiry

78573-45-2
312 suppliers
$10.00/1g